Question: Please read through all questions before starting work 1) Consider Figure 1.65b (pp92) in the text. What would the % Copper be in the liquid

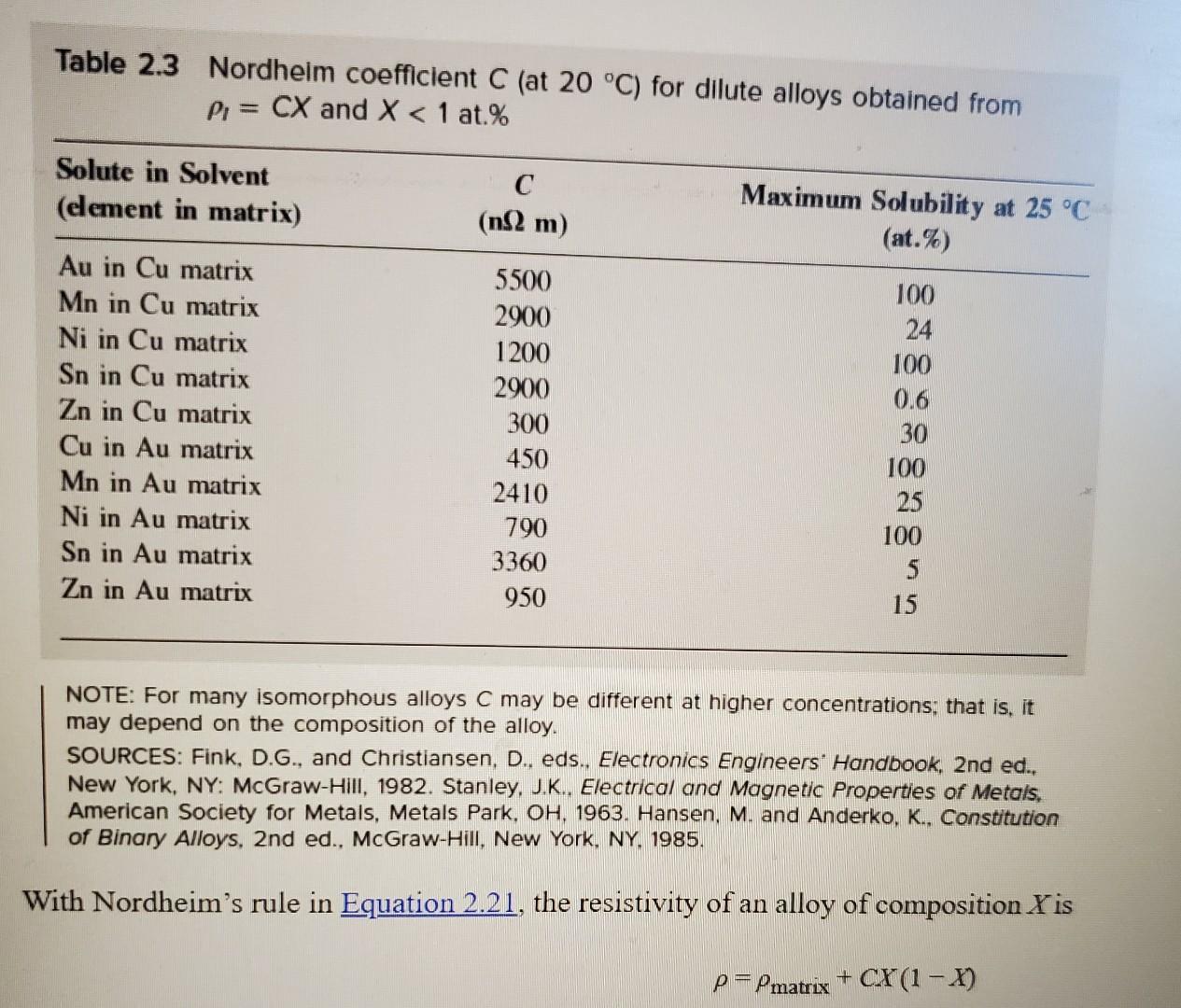
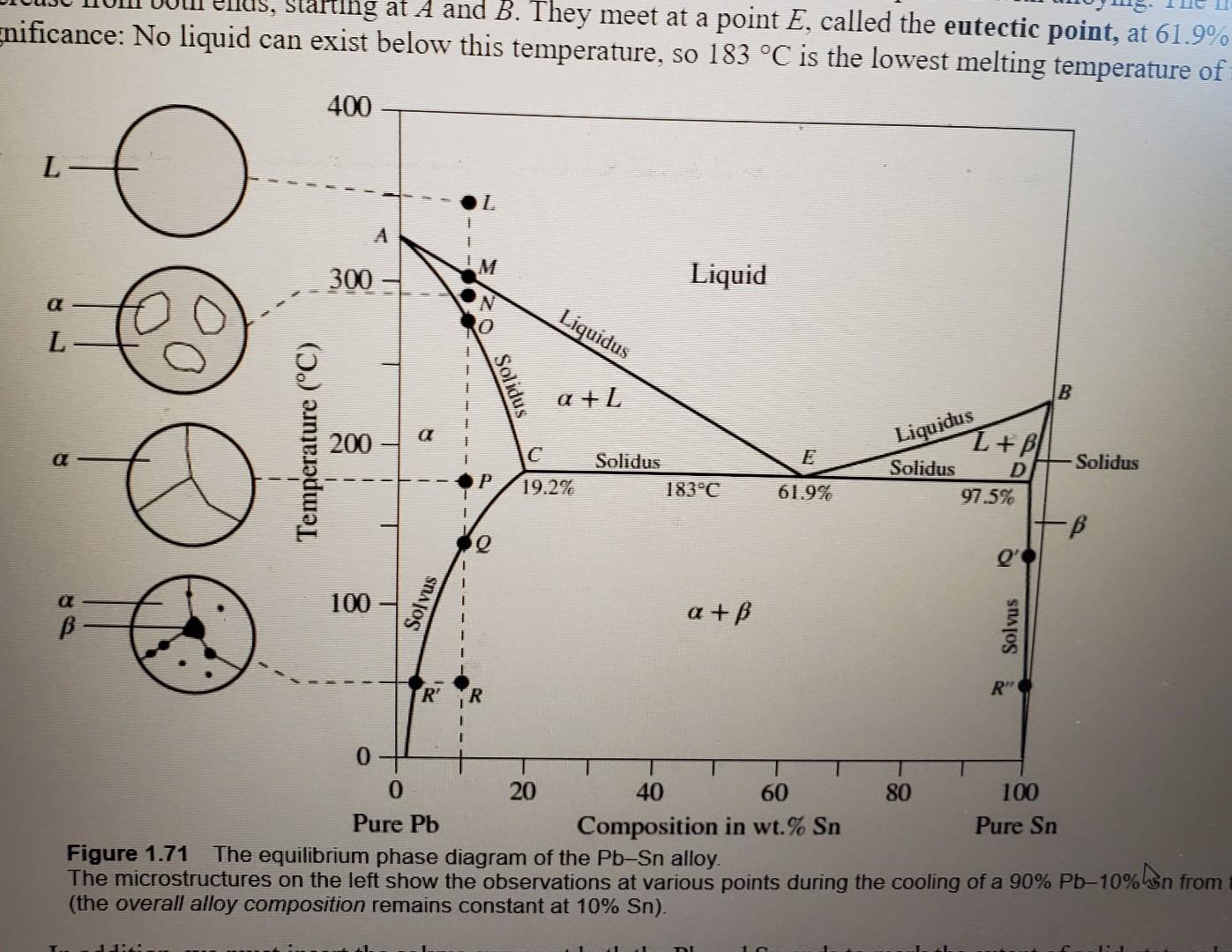
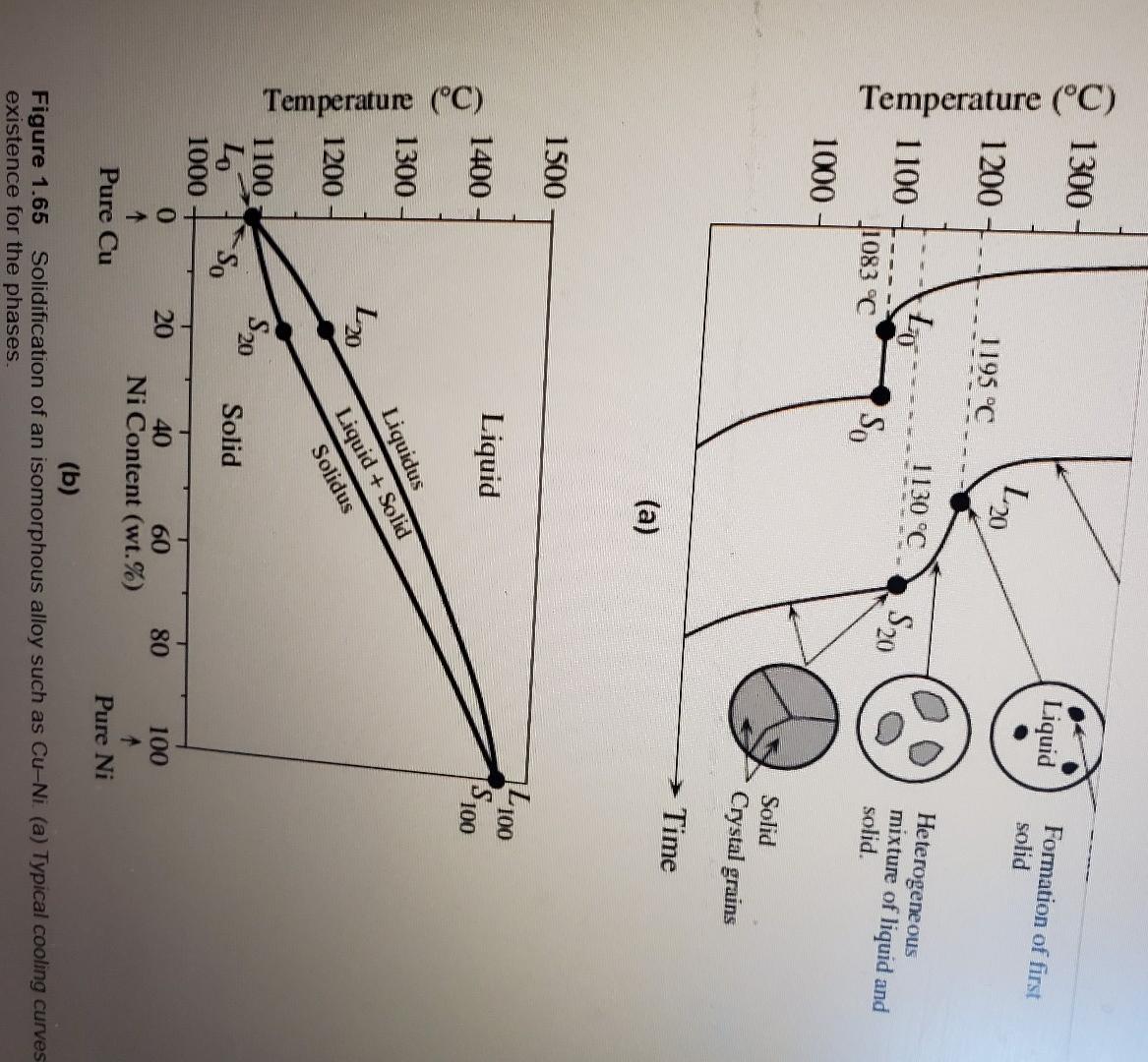
Please read through all questions before starting work 1) Consider Figure 1.65b (pp92) in the text. What would the % Copper be in the liquid and the solid given an overall Nickel concentration of 50% at the following temperatures: a) 1100C b) 1280C c) 1450C 2) Consider part b of question 3. Assuming values of 20% for the concentration of Nickel in the liquid and 70% for the concentration of Nickel in the solid, determine the weight percent of the solid and liquid. 3) Consider Figure 1.71(pp98) in the text. What would the \% Lead be in the liquid, the solid, and the solid given an overall Tin concentration of 30% at the following temperatures: a) 100C b) 225C c) 300C 4) Consider part a of question 3. Assuming values of 10% for the concentration of Tin in the solid and 90% for the concentration of Tin in the solid, determine the weight percent of the solid and solid. 5) Referring to Figure 1.71 (pp98) in the text, if we consider a Tin concentration of 61.9%, is there any temperature at which there is a will exist a mixture of liquid and solid? If so, what temperature. If not, why? 6) Consider a bar of aluminum with a mobility of 50cm2/Vs and an electron concentration of 1.811023cm3. What value of electric field must be applied to see a current density of 0.5Acm2? 7) Consider table 2.3 (pp147). Calculate the total resistivity for a mixture of Manganese (Mn) in Copper (Cu). If the mixture is not valid due to solubility, simply enter 'Not Valid'. You may assume a resistivity of Copper at 17.2nm. a) 0%Mn b) 10%Mn c) 20%Mn d) 30%Mn Table 2.3 Nordheim coefficient C (at 20C ) for dilute alloys obtained from l=CXandX
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


