Question: Answer question based on above info. A solid mixture consists of 47.6 g of KNO3 (potassium nitrate) and 8.4 g of K2SO4 (potassium sulfate). The
Answer question based on above info.
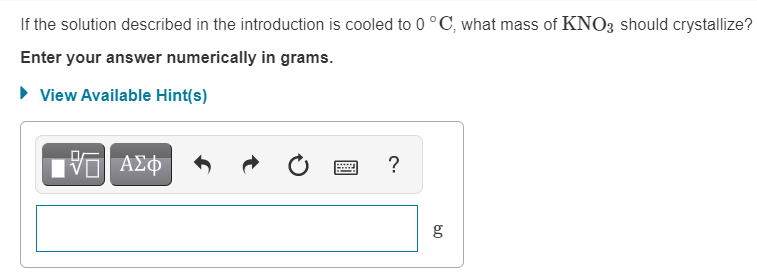
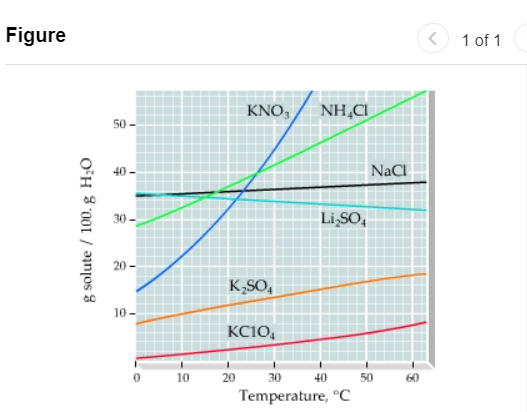
A solid mixture consists of 47.6 g of KNO3 (potassium nitrate) and 8.4 g of K2SO4 (potassium sulfate). The mixture is added to 130.g of water Use this solubility curve (Figure 1) to answer the questions. Figure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


