Question: Answer Question (BOX ANSWER) Acetylene and oxygen react to form carbon dioxide and water, like this: 2C,H,()+502(E) 400,(2) +2H20 (E) )+) Suppose a mixture of
Answer Question (BOX ANSWER)
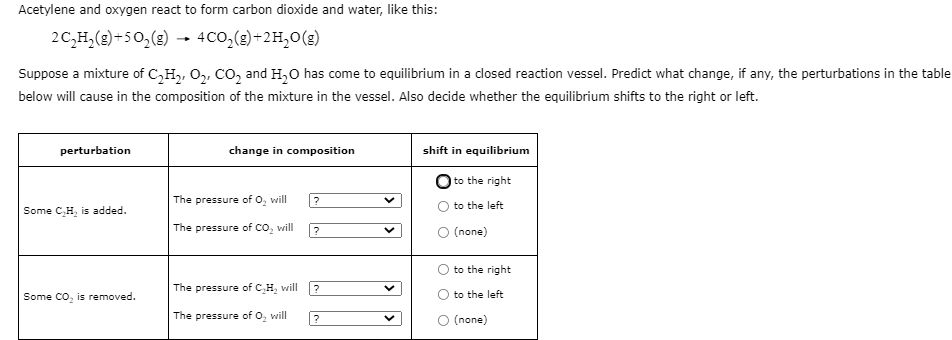
Acetylene and oxygen react to form carbon dioxide and water, like this: 2C,H,()+502(E) 400,(2) +2H20 (E) )+) Suppose a mixture of C_Hz, , COand H20 has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation change in composition shift in equilibrium The pressure of o, will to the right to the left ? Some CH is added. The pressure of Co, will ? O (none) to the right The pressure of CH will ? V Some Co, is removed. to the left The pressure of o, will ? O (none)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


