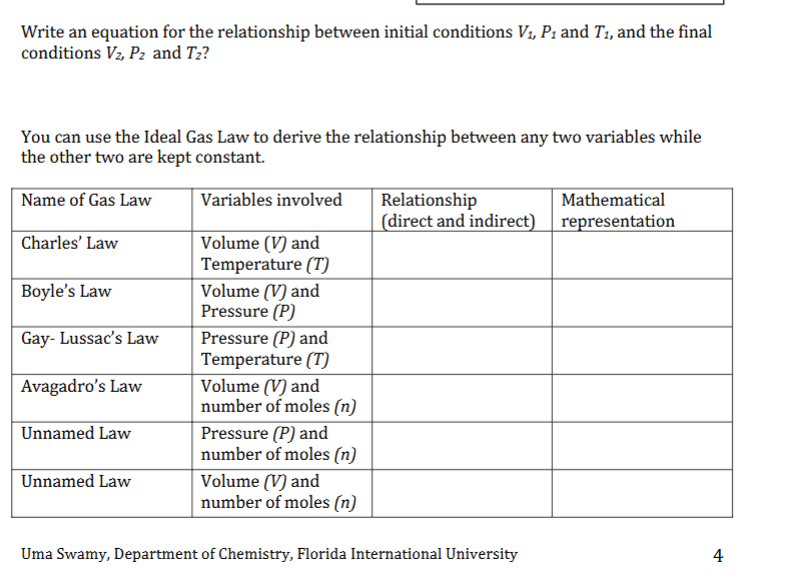
Question: answer questions short Write an equation for the relationship between initial conditions V3, P; and T3, and the final conditions Vz, Pz and T2? You
answer questions short
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


