Question: Answer quickly for thumbs up Answer is Methane (CH4) can be dehydrogenated according to the following reaction - 2CH4(g)C2H2(g)+3H2(g) However, the acetylene (C2H2) produced can
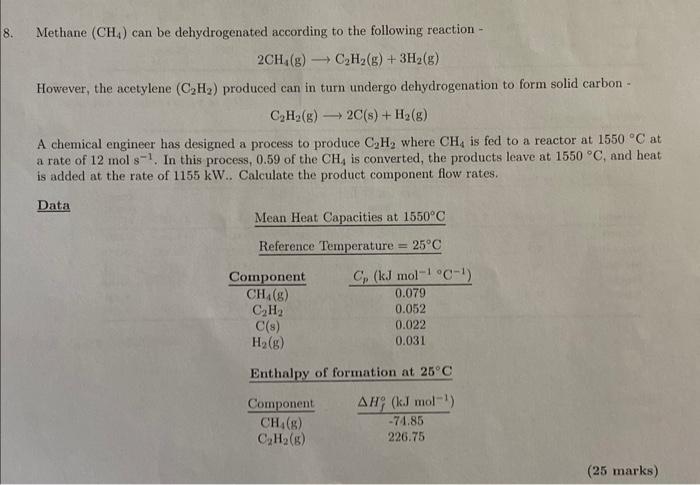
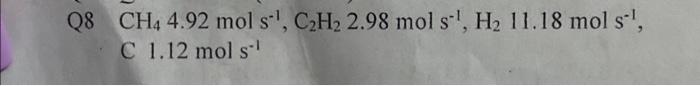
Methane (CH4) can be dehydrogenated according to the following reaction - 2CH4(g)C2H2(g)+3H2(g) However, the acetylene (C2H2) produced can in turn undergo dehydrogenation to form solid carbon - C2H2(g)2C(s)+H2(g) A chemical engineer has designed a process to produce C2H2 where CH4 is fed to a reactor at 1550C at a rate of 12mols1. In this process, 0.59 of the CH4 is converted, the products leave at 1550C, and heat is added at the rate of 1155kW.. Calculate the product component flow rates. Q8 CH44.92mols1,C2H22.98mols1,H211.18mols1, C 1.12mols1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


