Question: answer sheet, all data given Recorded Data Mass of Crucible Mass of Crucible and Hydrated Sample Mass of Crucible and Dehydrated Sample Mass of Empty
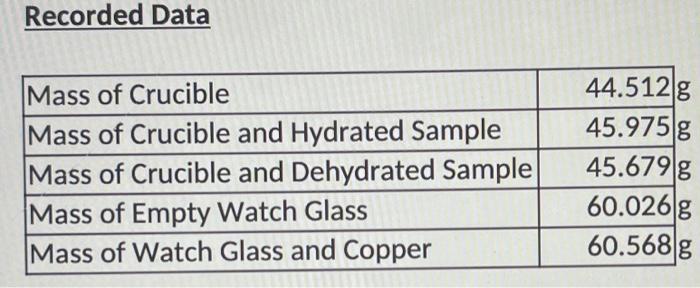


Recorded Data Mass of Crucible Mass of Crucible and Hydrated Sample Mass of Crucible and Dehydrated Sample Mass of Empty Watch Glass Mass of Watch Glass and Copper 44.512 g 45.975 g 45.679 g 60.026 g 60.568 g Recorded Data Mass of Crucible Mass of Crucible and Hydrated Sample Mass of Crucible and Dehydrated Sample Mass of Empty Watch Glass Mass of Watch Glass and Copper Calculated Data Mass of Hydrated Sample Mass of Dehydrated Sample Mass of Copper Number of Moles of Copper Mass of Water Number of Moles of Water Mass of Chlorine Number of Moles of Chlorine Mole Ratio, Chlorine : Copper Mole Ratio, Water: Copper in Hydrated Sample Formula of Dehydrated Sample (round ratio to nearest whole number) Formula of Hydrated Sample (round ratio to nearest whole number) In a certain compound of copper and oxygen, Cu Oy, we find that a sample weighing 0.6349 g contains 0.5639 g of Cu. What is the mole ratio (number of moles Cuumber of moles 0) in the sample? Write the resulting Empirical Formula. In a certain compound of chromium, chlorine, and water, Cr Clyz H2O, we find that a sample weighing 20.0000 g contains 3.9082 g of Cr and 8.1136 g of water. What is the mole ratio (number of moles Crumber of moles Cl and number of moles Crumber of moles of H20) in the sample? Write the resulting Empirical Formula. 3. A carbohydrate sample weighing 5.0000 g with a formula of CxHyoz is dehydrated to leave 2.0001 g of C. What is the mole ratio (moles of C/ moles of water) in the sample? Write the resulting Empirical Formula
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


