Question: Answer should be well explained. Propane (C3Hg) and air (21% O2 and 79% N2) burn at an air-fuel mass ratio of 20:1. Determine (a) the
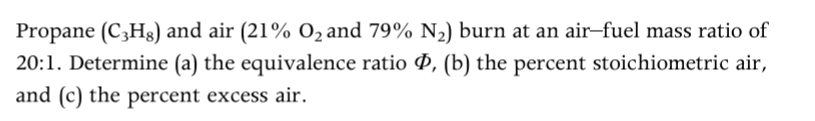
Answer should be well explained.
Propane (C3Hg) and air (21% O2 and 79% N2) burn at an air-fuel mass ratio of 20:1. Determine (a) the equivalence ratio 0, (b) the percent stoichiometric air, and (c) the percent excess air
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


