Question: Answer the following questions using the attached Excel graph and given equation of a line. 1. Determine the concentration of the solution if an absorbance

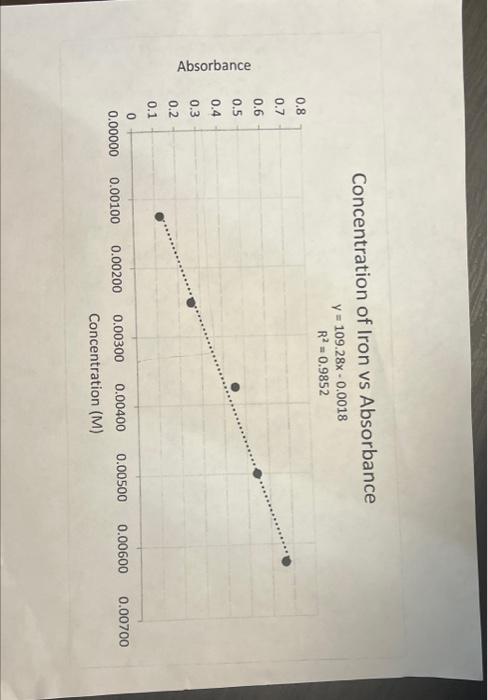
Answer the following questions using the attached Excel graph and given equation of a line. 1. Determine the concentration of the solution if an absorbance of .322 was measured using the graph. (2 points) a. Concentration of solution: 0.00 2. What is the slope of the line? Y=log.28y0.0.018 (1 point) 3. What is the y-intercept of the line? 0.0018 (1 point) 4. Calculate the concentration when the absorbance is .322 SHOW ALL WORK ! (3 points) 5. Compare your answers. Is one concentration value "better" than the other? Briefly describe Why? (3 points) Concentration of Iron vs Absorbance y=109.28x0.0018R2=0.9852
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


