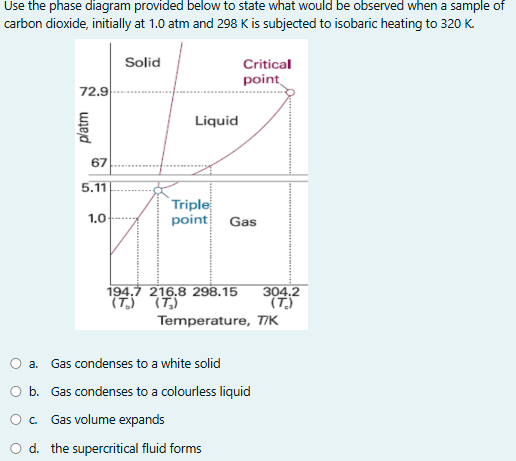
Question: answer the question Use the phase diagram provided below to state what would be observed when a sample of carbon dioxide, initially at 1.0 atm
answer the question
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


