Question: Answer the questions below about the highlighted atom in this Lewis structure: H :0: . H-0-0-0-H 1 H In how many sigma bonds does the
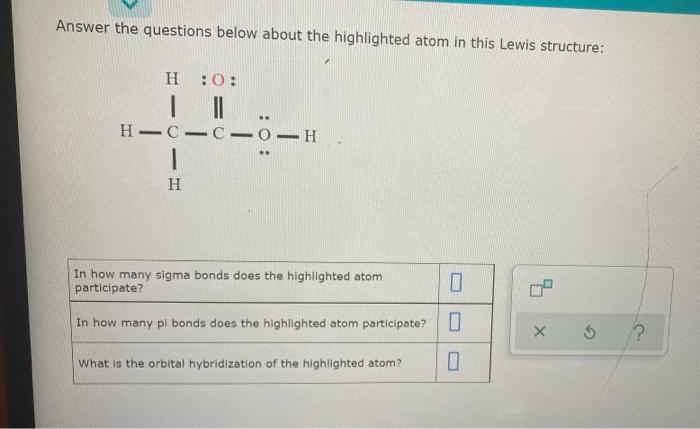
Answer the questions below about the highlighted atom in this Lewis structure: H :0: . H-0-0-0-H 1 H In how many sigma bonds does the highlighted atom participate? In how many pi bonds does the highlighted atom participate? 5 ? What is the orbital hybridization of the highlighted atom
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


