Question: Answer this: A reaction is said to have been completed if one of the reactants is completely consumed by the reaction. In this experiment, sodium


Answer this:
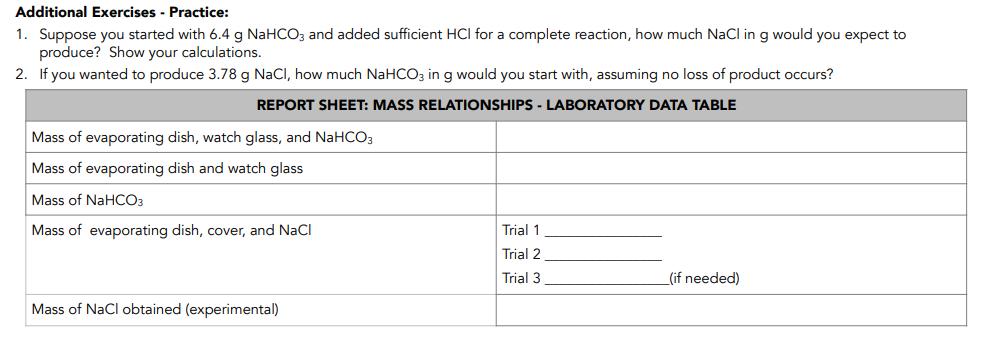
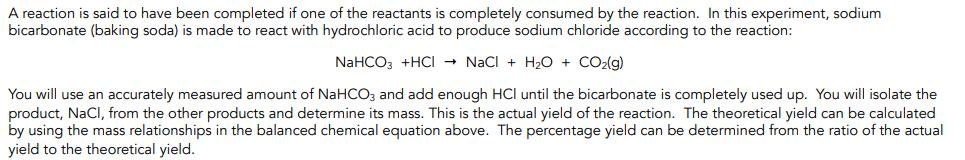
A reaction is said to have been completed if one of the reactants is completely consumed by the reaction. In this experiment, sodium bicarbonate (baking soda) is made to react with hydrochloric acid to produce sodium chloride according to the reaction: NaHCO3 +HCI - NaCI + H2O + CO2(g) You will use an accurately measured amount of NaHCOz and add enough HCI until the bicarbonate is completely used up. You will isolate the product, NaCl, from the other products and determine its mass. This is the actual yield of the reaction. The theoretical yield can be calculated by using the mass relationships in the balanced chemical equation above. The percentage yield can be determined from the ratio of the actual yield to the theoretical yield.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


