Question: answer this please :) For the reaction below, Kc=0.154 at 2000K. 2CH4(g)C2H2(g)+3H2(g) A 1.75L equilibrium mixture at 2000K contains 0.350 mol each of CH4(g) and
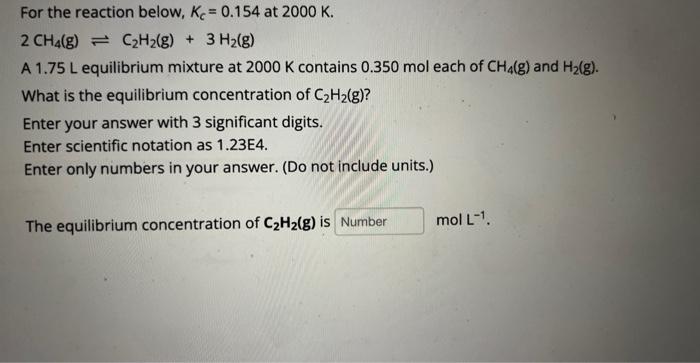
For the reaction below, Kc=0.154 at 2000K. 2CH4(g)C2H2(g)+3H2(g) A 1.75L equilibrium mixture at 2000K contains 0.350 mol each of CH4(g) and H2(g). What is the equilibrium concentration of C2H2(g) ? Enter your answer with 3 significant digits. Enter scientific notation as 1.23E4. Enter only numbers in your answer. (Do not include units.) The equilibrium concentration of C2H2(g) is molL1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


