Question: answer this please. this's the last attempt + also attached part D 1. In part D of this experiment, which of the following are the
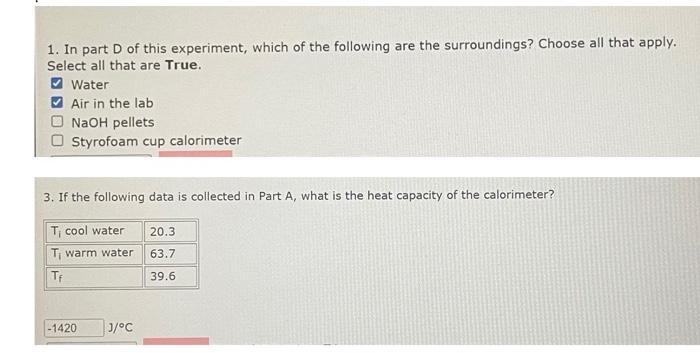

1. In part D of this experiment, which of the following are the surroundings? Choose all that apply. Select all that are True. Water Air in the lab NaOH pellets Styrofoam cup calorimeter 3. If the following data is collected in Part A, what is the heat capacity of the calorimeter? CAUTION: Solid NaOH pellets used in Parts D \& E absorb water from the air and form a slippery solution that is very corrosive. Handle the pellets with care. Clean up any spilled NaOH(s) and be sure the cover is on the jar after taking what you need. If NaOH comes in contact with skin flush immediately with water until the slippery feeling is gone. Part D: The Enthalpy of Solution of NaOH(s) Place 50.0mL of distilled water in the reaction calorimeter. Measure its temperature. Weigh about 2.00g(0.05mol) of NaOH(s) in a dry 50mL beaker. The approximate number of NaOH(s) pellets to count out will be displayed on the sign outside the balance room. Add NaOH(s) to the water, stirring to dissolve. Record the highest temperature reached. Is this an endothermic or exothermic reaction? Once the measurement has been recorded, add 50mL of 1.0MHCl and then pour down the sink with tap water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


