Question: Answer using the figure below for the liquid phase reaction A, B CpA=Cpg= 50 J/mol.K, the feed is pure A and the heat exchanger is
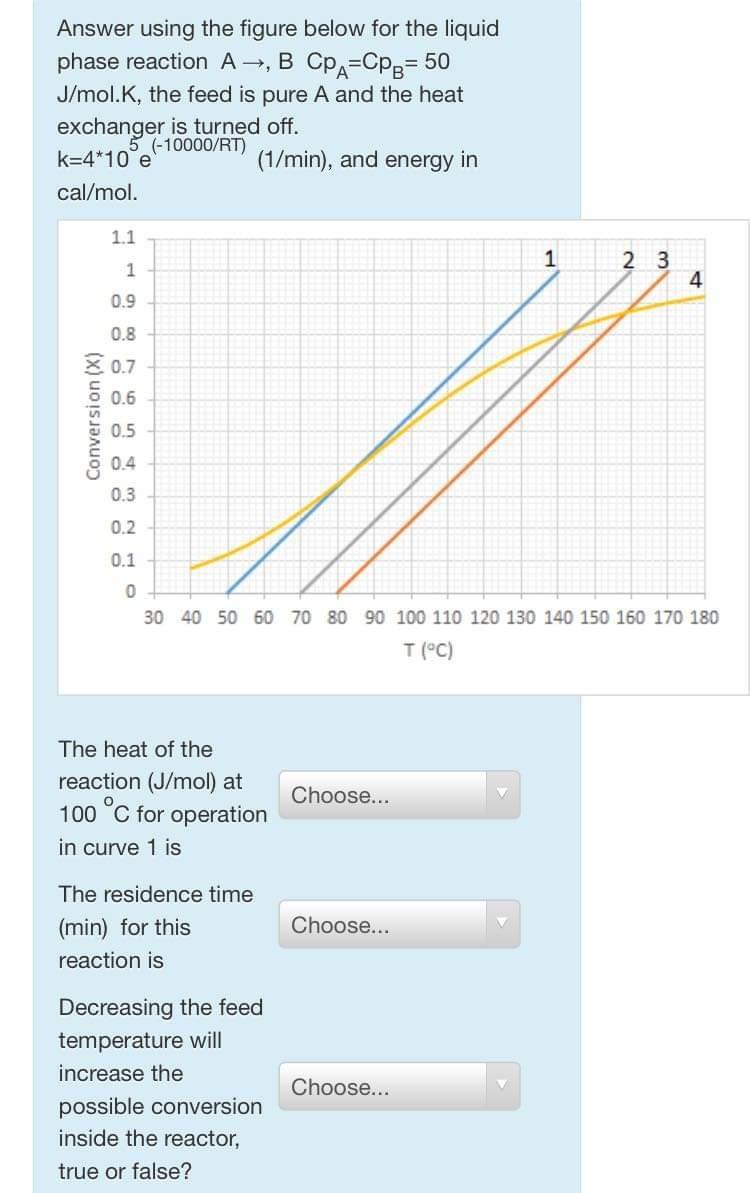
Answer using the figure below for the liquid phase reaction A, B CpA=Cpg= 50 J/mol.K, the feed is pure A and the heat exchanger is turned off. (-10000/RT) k=4*10 e (1/min), and energy in cal/mol. 11 1 2 3 1 4 0.9 0.8 0.7 0.6 Conversion (X) 0.5 0.4 0.3 0.2 0.1 0 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 T (C) The heat of the reaction (J/mol) at 100C for operation in curve 1 is Choose... The residence time (min) for this reaction is Choose... Decreasing the feed temperature will increase the possible conversion inside the reactor, true or false? Choose
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


