Question: answers given -> show the work Voltaic cells (Galvanic cells) NOTE: The answers to EMF calculations are VERY sensitive to the values of the reduction
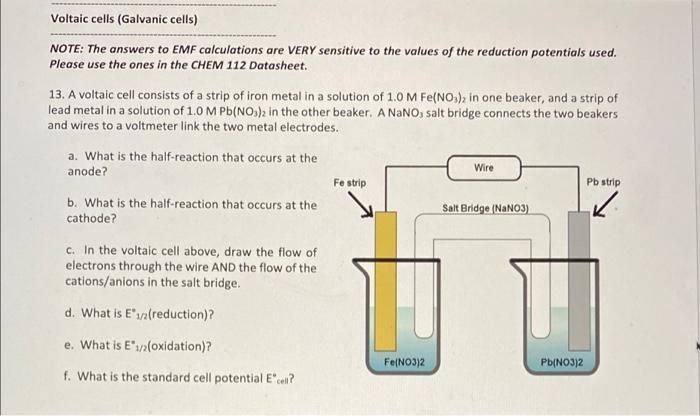
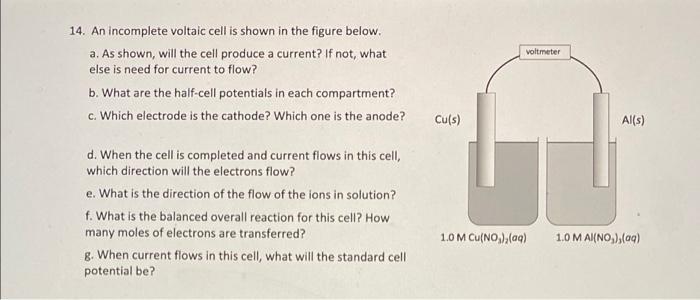
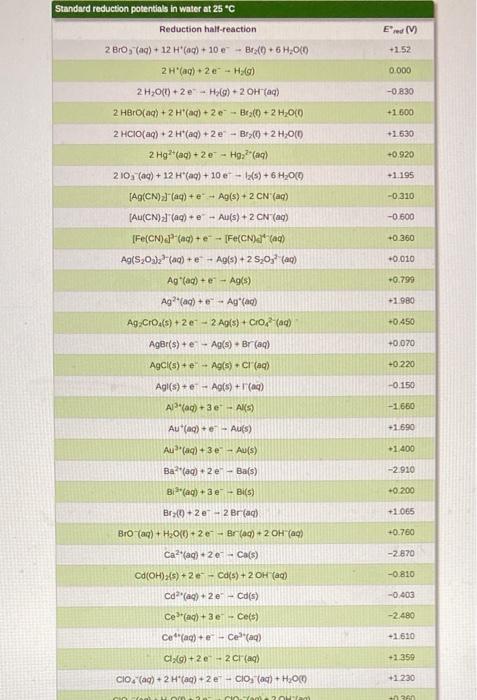
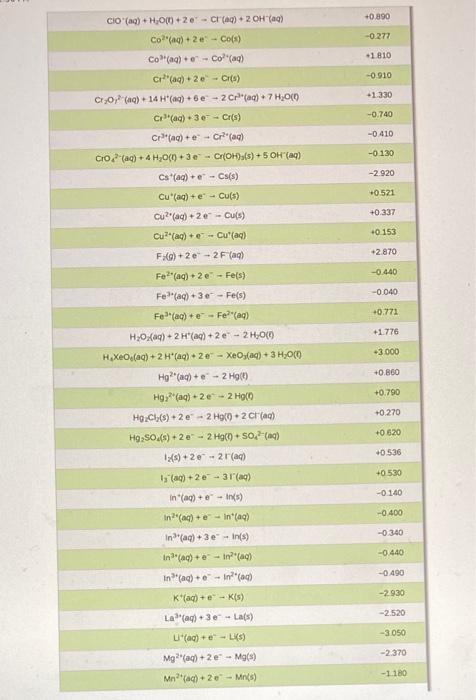
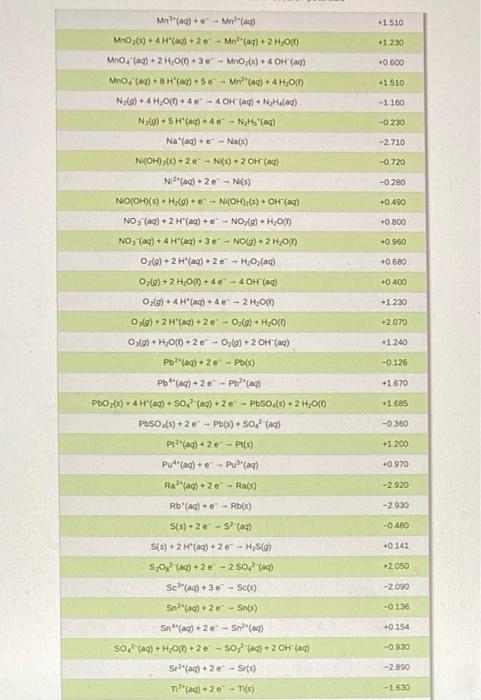


Voltaic cells (Galvanic cells) NOTE: The answers to EMF calculations are VERY sensitive to the values of the reduction potentials used. Please use the ones in the CHEM 112 Datasheet. 13. A voltaic cell consists of a strip of iron metal in a solution of 1.0 M Fe(NO) in one beaker, and a strip of lead metal in a solution of 1,0 M Pb(NO3)2 in the other beaker. A NaNO, salt bridge connects the two beakers and wires to a voltmeter link the two metal electrodes. a. What is the half-reaction that occurs at the anode? b. What is the half-reaction that occurs at the Salt Bridge (NaNO3) cathode? Wire Fe strip Pb strip K C. In the voltaic cell above, draw the flow of electrons through the wire AND the flow of the cations/anions in the salt bridge d. What is E*: (reduction)? e. What is E 1/2(oxidation)? Fe(NO3)2 Pb(NO3)2 f. What is the standard cell potential Ecl? voltmeter 14. An incomplete voltaic cell is shown in the figure below. a. As shown, will the cell produce a current? If not, what else is need for current to flow? b. What are the half-cell potentials in each compartment? c. Which electrode is the cathode? Which one is the anode? Cu(s) Al(s) d. When the cell is completed and current flows in this cell, which direction will the electrons flow? e. What is the direction of the flow of the ions in solution? f. What is the balanced overall reaction for this cell? How many moles of electrons are transferred? 8. When current flows in this cell, what will the standard cell potential be? 1.0 M Cu(NO),(aq) 1.0 M AI(NO),(09) Er(V +1.52 Standard reduction potentials in water at 25C Reduction half-reaction 2B0, (aq) + 12 H*(aq) + 10 - Brx(+6,00 2H*(aq) +20-HC) 2H,00) +2e H_() + 2OH(aq) 2 HBrO(aq) + 2 H(aq) + 2e - Braco + 2 H2O(0 0.000 -0.8:30 +1.600 -1.630 2 HCIO(aq) + 2 H(aq) +2e - Br(+2H,000 2 Hg2+ (aq) +2e - H922(aq) 210, (aq) + 12 H(aq) + 10 e - 12(s) + 6H2010 +0.920 +1.195 (ACN))() + - Ag(s) + 2 CN (aq) -0.310 -0.500 JAU(CN)"(aq) + e -- Au(s) + 2 CN"(aq) [Fe(CN) -(ac)+ e -- [Fe(CN)a' (aq) Ag(S03)2(aq) + e - Agls) + 2 520,? () +0.360 +0.010 +0.799 +1.980 Ag (ac) + e - Ag(s) Ag?(a) + Ag (ac) Ag Cros(s) +20-2A(s) + Croad) Ager(s) + e - Ag(s) - Br(aq) AgCl(s) + e - Agls). Cl(aq) +0450 -0.070 -0220 Agl() +1A015) + () -0.150 Apaq) + 3 e - Al(s) -1660 Au(aq) + Aus) +1.690 -1.400 Au(aq) + 3e - Au(s) -2.910 Ba?(aq) + 2 e-- Bas) Bi (aq) +3e - Bi(s) +0.200 Br -2e - 2 Br(aq) +1.065 Bro(aq) + H2O(0) + 2e - Br() + 2 OH (0) +0.760 Ca2(aq) +20+ Ca(s) -2.870 -0.810 Ca(OH)>(s) + 2e - Cd(s) + 2 OH (c) Ca(aq) + 2e - Cd(s) Ceac) +3e - Ce(s) -0.403 -2.480 ce(a) +- Celaq) -1.610 CI,Co)+2e-2 craq) +1.359 CIO(aq) + 2 H(aq) + 2e - CIO (aq) + H2000 +1230 2 - +0.890 CIO (ac) + H200 +20 - (aq) + 2 OH(a) Co(aq) + 2e - Co(s) -0.277 1 810 Co (aq) + Col(aq) -0.910 Cr?"(aq) + 2 C) CT20, (aq) + 14 H(aq) + 6e- 2 Crac) + 7 H2O(0) +1.330 -0.740 -0 410 -0 130 -2.920 Cr(aq) 3 - Cr(s) Crac) + Crac) CO2(aq) + 4H2O(+30 - Cros(s) + 5 OH(aq) Cs(ag) - Cs(s) Cu(c) + e - Cu(s) Cu?"(aq) +2 -- Cu(s) Cu (aq) + Cu(aq) -0.521 +0.337 40 153 +2.870 F:() 2e-2 F (aq) Fe2(aq) + 2 e Fe(s) -0.440 -0.040 Fe(aq) +30 - Fe(s) +0.771 Fe(a) Fel(aq) +1.776 H2O) + 2 Hag) 2 - 2 HOC H Xeo.(aq) + 2H(aq) + 20 - XeOs(aq) + 3 H2000 -3000 Hg (aq) +- 2 H 0.860 +0.790 Hg, (aq) 2e-2 HOCO +0.270 Ho Ch(s) + 2 e 2 Holo.2 crac) H92SO4() +2e -- 2 Haco +50 (0) 1(s) +2e-2 (ac) + +0620 +0.536 +0.530 1s (0) +2e - 31(c) -0.140 in (aq) +-In() In(aq) +-In(a) -0.400 -0.340 -0.440 In(aq) + 3eIn(s) In"(aq) +-in"(a In"(ac) +- In?" K'(aq) + e-K() -0490 -2930 -2.520 Lala) 3e Las) -3.050 -2.370 L'(aq) + e-LK) Mg(ac) +2e-Mg(s) Mnach 20 - Mn(s) -1.180 1510 1 230 +0600 -1510 -1180 -0230 -2710 -0.720 -0280 +0.490 +0000 +0.960 Mn) - Mn?) MO(s) + 4H(aq) + 20 - Mnaq) + 2H,00 Mnog) + 2 H2O(0) 3 - MO(s) 4 OH() Mno(a) BH (0) 50 - Malac) + 4H,000 N:o) + 4H,000. 40 - 4 OH (c) + N Head) N:(0)5 H(aq) + 47 - NH (4) Naag). Nas) N(OHxs) + 2e - N() + 2OH(a) N'(ac) +2e-N() NO(OH)(s) - H (0)N(OH):(8) + OH(aq) NO, (aq) + 2H"(aq) + NO_(Q)+H300 NO, (ac) + 4H(aq) + 3e - NOG).2H00 (0)2(aq) 2e-H2O,(ac) 0,()+2H,00) + 4 - 4 OH(aq) OX) + 4H*(aq) + 48 - 21.00 05(0)2 H'(aq) + 2e-O(g) + H2000 050)+H70(+2e-00) + 2 OH(a) Pb (0)20 - Pb(s) Pb (04) -2e - Podaci PbO2(s) 4 "(0) +SO) 2e-PbSO4)2 H,000 Pbso() 2e - Pb(s) Soa) Pr?"() +20 - Pt(s) Put (aq) + (2) Ra(aq) + 2e - Ras) +0.680 +0.400 +1230 +2070 +1240 -0126 +1670 -1.685 -0.360 +1.200 +0.970 -2.920 -2.930 -0480 +0.141 *2050 Rb (aq) + e - Ris) S(s) - 20-SP (a) S(5) + 2 H+ (aq) + 2 H2SC) SO (a) +20 - 250 (0) Schlag) + 3e - Sc(s) Sna) 2e - Sn(s) Sn") + 2e - Snac) so (a) + H,000 -2e - Soyad) - 20H () -2090 -0136 -0.154 -0.930 Sr2(aq) + 2 -Sr(s) -2 890 Tif(aq) + 2e - Tis) -1630 -0.370 Ti**(aq) + e- Ti2(aq) T14*(aq) + e Ti3(aq) 0.000 TI*(aq) + e - Tl(s) -0.340 -1.790 U3+ (aq) + 3 e - U(s) U**(aq) + e - U3+ (aq) V2+ (aq) + 2 e V(s) -0.610 -1.190 Zn2+(aq) + 2 e - Zn(s) -0.763 - -> 13. a. Anode: Fe(s) Fe(aq) + 2e b. Cathode: Pb2+(aq) + 2e Pb(s) C. electrons flow from anode (Fe) to cathode (Pb). Anions in salt bridge flow towards the anode (Fe) and cations in the salt bridge flow towards the cathode (Pb). d. Ered=-0.126 V e. Eox = 0.440 V f. Ecell = 0.314 V 14. a. The cell will not produce a current because the circuit is not complete. A salt bridge is needed to provide a means for ions to flow, completing the circuit and maintaining charge neutrality in the cells. b. Cu?" (aq) + 2 e Cu(s) Ered=0.337 V; Al(s) Alt(aq) + 3 e Ex = 1.66V c. The copper electrode is the cathode. The aluminum electrode is the anode. d. Electrons flow through the wire from the Al electrode to the Cu electrode. e. The copper cations flow from the electrolyte solution to the copper cathode. The aluminum cations are produced at the aluminum anode and go into the electrolyte solution. f. 3 Cu?"(aq) + 2 Al(s) 3 Cu(s) + 2 Alaq) There are 6 moles of electrons transferred. 8. E' cell = 1.997 v ->
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


