Question: Antacid Dry Lab Data (Titration 2) Sample Number Trial 1 Trial 2 Unit 0.1287 M Molarity of NaOH solution Molarity of HCl solution 0.1107 M
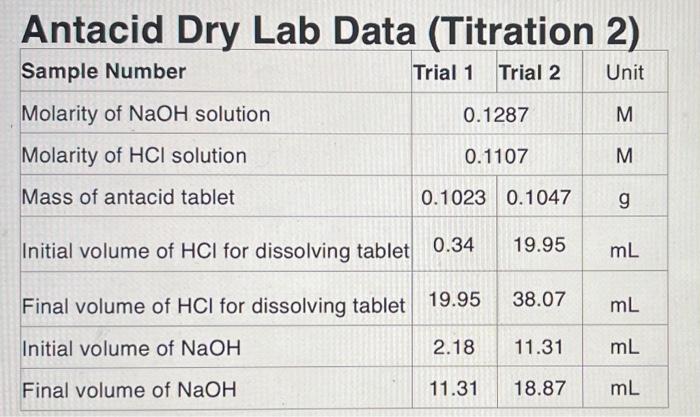
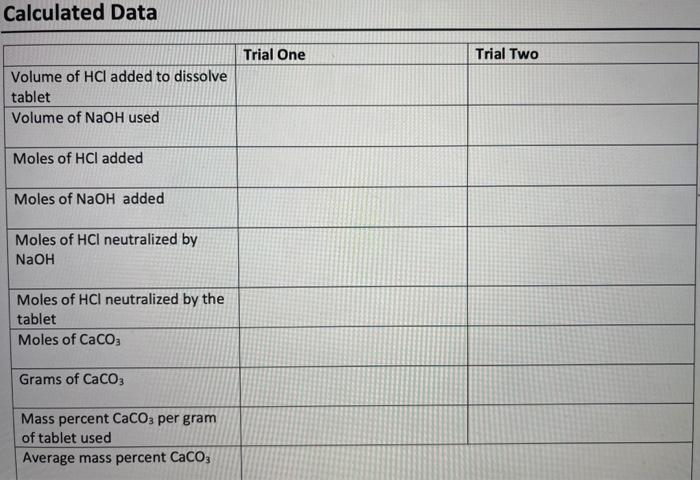
Antacid Dry Lab Data (Titration 2) Sample Number Trial 1 Trial 2 Unit 0.1287 M Molarity of NaOH solution Molarity of HCl solution 0.1107 M Mass of antacid tablet 0.1023 0.1047 g Initial volume of HCl for dissolving tablet 0.34 19.95 mL Final volume of HCl for dissolving tablet 19.95 38.07 mL Initial volume of NaOH 2.18 11.31 mL Final volume of NaOH 11.31 18.87 mL Calculated Data Trial One Trial Two Volume of HCI added to dissolve tablet Volume of NaOH used Moles of HCl added Moles of NaOH added Moles of HCl neutralized by NaOH Moles of HCl neutralized by the tablet Moles of CaCO3 Grams of CaCO3 Mass percent CaCO3 per gram of tablet used Average mass percent CaCO3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


