Question: Calculate Sheet, data given - Sample Number Mass of KHP Molarity of HCl solution Initial volume of HCl solution Final volume of HCl solution Initial
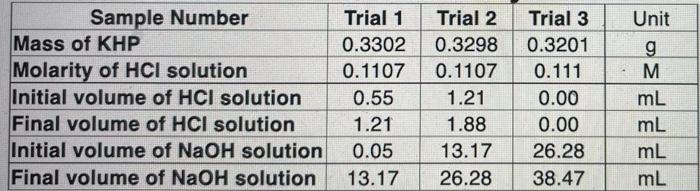
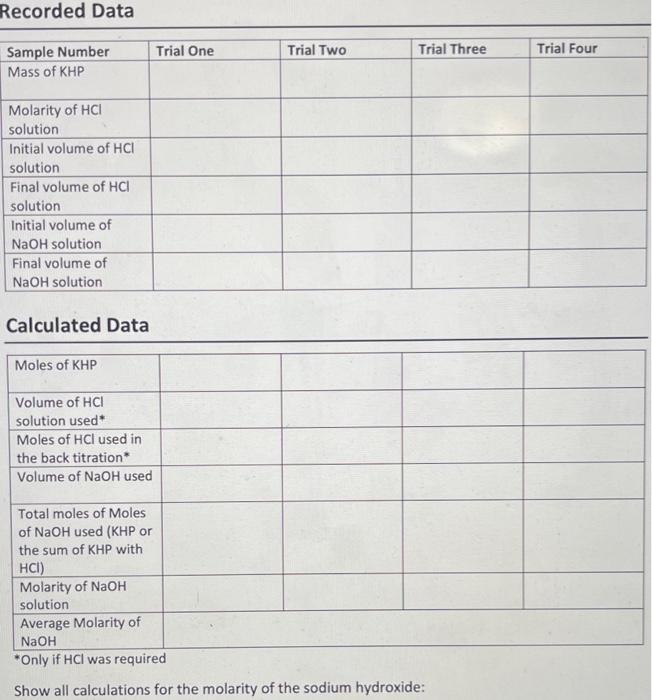

- Sample Number Mass of KHP Molarity of HCl solution Initial volume of HCl solution Final volume of HCl solution Initial volume of NaOH solution Final volume of NaOH solution Trial 1 0.3302 0.1107 0.55 1.21 0.05 13.17 Trial 2 0.3298 0.1107 1.21 1.88 13.17 26.28 Trial 3 0.3201 0.111 0.00 0.00 26.28 38.47 Unit g M mL mL mL mL Recorded Data Trial One Trial Two Trial Three Trial Four Sample Number Mass of KHP Molarity of HCI solution Initial volume of HCI solution Final volume of HCI solution Initial volume of NaOH solution Final volume of NaOH solution Calculated Data Moles of KHP Volume of HCI solution used Moles of HCl used in the back titration Volume of NaOH used Total moles of Moles of NaOH used (KHP or the sum of KHP with HCI) Molarity of NaOH solution Average Molarity of NaOH *Only if HCl was required Show all calculations for the molarity of the sodium hydroxide: 1. Would adding extra deionized water to the Erlenmeyer flask during the titration affect the molarity of the NaOH solution? Explain your reasoning. 2. Why is it important that air bubbles from the buret or valve are removed prior to starting the titration? 3. Why is it proper to read the initial volume of the buret instead of trying to adjust the volume to a round number such as 0 mL? 4. Calculate the molarity of a NaOH solution if 23.25 mL of NaOH is titrated with 0.475 grams of KHP and 1.25 ml of 0.100 M HCl is required
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


