Question: any help? Standard solution 'ii' was prepared by placing 250L of a 100mgL1 stock magnesium solution into a 100cm3 volumetric flask and prepared to volume
any help? 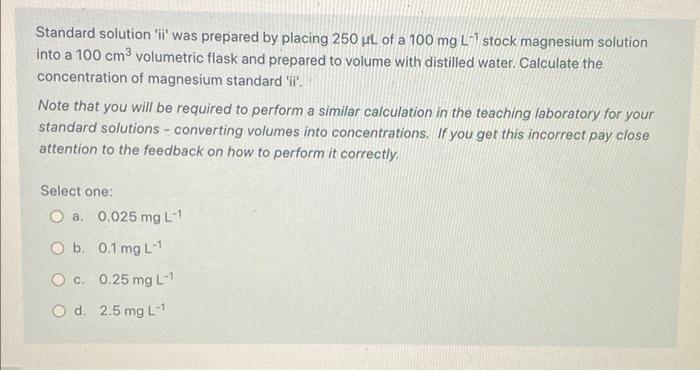

Standard solution 'ii' was prepared by placing 250L of a 100mgL1 stock magnesium solution into a 100cm3 volumetric flask and prepared to volume with distilled water. Calculate the concentration of magnesium standard 'ii'. Note that you will be required to perform a similar calculation in the teaching laboratory for your standard solutions - converting volumes into concentrations. If you get this incorrect pay close attention to the feedback on how to perform it correctly. Select one: a. 0.025mgL1 b. 0.1mgL1 c. 0.25mgL1 d. 2.5mgL1
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


