Question: Apply the Skill 18.29 We will see In Chapter 20 that derivatives of aniline will react with aryl chlorides (RCOCI) in the presence of pyridine
Apply the Skill 18.29 We will see In Chapter 20 that derivatives of aniline will react with aryl chlorides (RCOCI) in the presence of pyridine (a base) to yield aromatic amides, as shown below for the parent aniline: 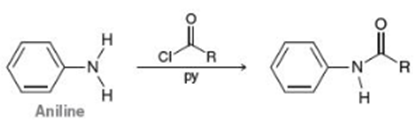
The drug flutamide, sold under the trade name Eulexin, Is used to treat prostate cancer by decreasing the action of male hormones. Starting with (trifluoromethyl)benzene, propose a synthesis of flutamide.(J. Med. Chem. 1967, 10, 93-95.) Note: You will need to use the amide-forming reaction above. 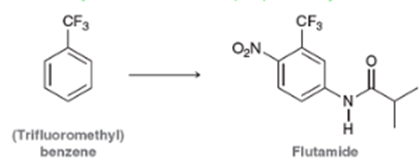
a.
Your answer is partially correct. Try again. We begin by considering which of these two groups should be installed last. The nitro group is an electron withdrawing substituent and a(n) meta director The amide group is an electron-donating substituent and a(n) i I-director. Therefore, it makes sense for the last step of our synthesis to be nucleophIlIc aryl substil In this way, the amide group directs ortho,para : the final step to occur in the desired location ( para'Ito the large amide group).
Aniline H H CI py R N H R CF3 (Trifluoromethyl) benzene ON. CF3 Z-I Flutamide
Step by Step Solution
3.51 Rating (151 Votes )
There are 3 Steps involved in it
To synthesize flutamide starting from trifluoromethylbenzene follow these steps Step 1 Nitration Int... View full answer

Get step-by-step solutions from verified subject matter experts


