Question: Approximately how much do the following enzymesdecrease the activation energy (G) of the reactions they catalyze? An aminoglycoside complexed with copper has been shown to

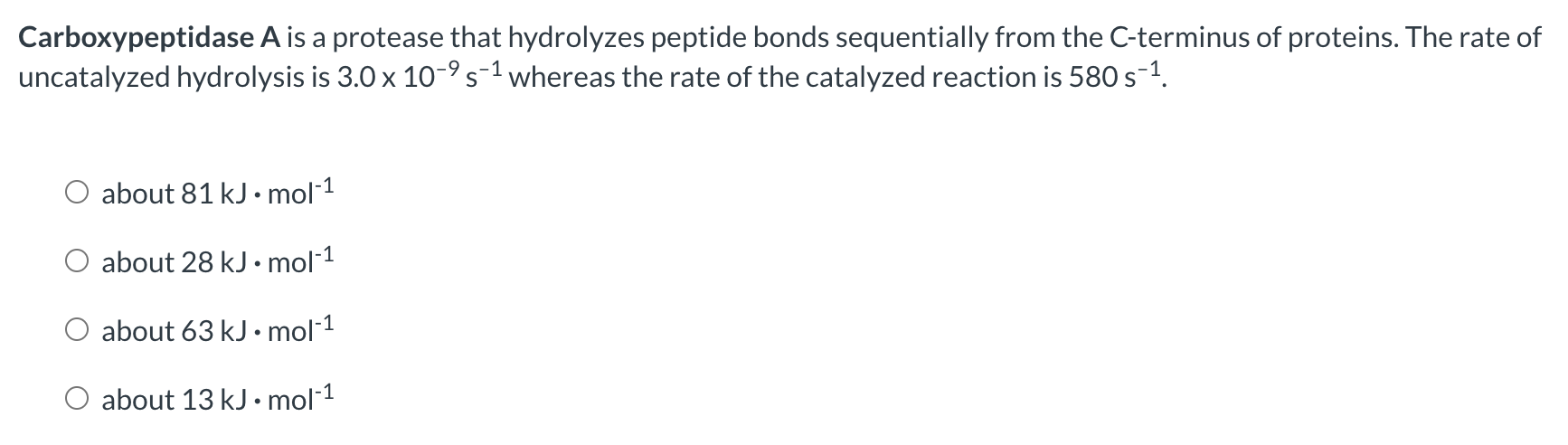
Approximately how much do the following enzymesdecrease the activation energy (G) of the reactions they catalyze? An aminoglycoside complexed with copper has been shown to cleave DNA and can potentially be used as an antitumor agent. The glycoside cleaves DNA at a rate of 3.57h1 whereas the uncatalyzed rate of DNA hydrolysis is 3.6108h1. about 25kJmol1 about 77kJmol1 about 69kJmol1 about 46kJmol1 Carboxypeptidase A is a protease that hydrolyzes peptide bonds sequentially from the C-terminus of proteins. The rate of uncatalyzed hydrolysis is 3.0109s1 whereas the rate of the catalyzed reaction is 580s1. about 81kJmol1 about 28kJmol1 about 63kJmol1 about 13kJmol1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


