Question: Aproducts,r=kCA5/2 occurs in a steady state CSTR with 80% conversion of A. Let the space time for the CSTR be 1 (a). By solving the
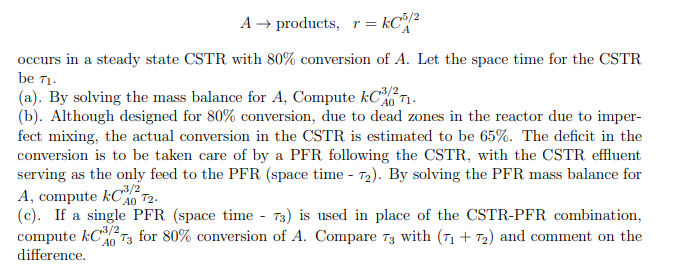
Aproducts,r=kCA5/2 occurs in a steady state CSTR with 80% conversion of A. Let the space time for the CSTR be 1 (a). By solving the mass balance for A, Compute kCA03/21 (b). Although designed for 80% conversion, due to dead zones in the reactor due to imperfect mixing, the actual conversion in the CSTR is estimated to be 65%. The deficit in the conversion is to be taken care of by a PFR following the CSTR, with the CSTR effluent serving as the only feed to the PFR (space time 2 ). By solving the PFR mass balance for A, compute kCA03/22 (c). If a single PFR (space time 3 ) is used in place of the CSTR-PFR combination, compute kCA03/23 for 80% conversion of A. Compare 3 with (1+2) and comment on the difference. Aproducts,r=kCA5/2 occurs in a steady state CSTR with 80% conversion of A. Let the space time for the CSTR be 1 (a). By solving the mass balance for A, Compute kCA03/21 (b). Although designed for 80% conversion, due to dead zones in the reactor due to imperfect mixing, the actual conversion in the CSTR is estimated to be 65%. The deficit in the conversion is to be taken care of by a PFR following the CSTR, with the CSTR effluent serving as the only feed to the PFR (space time 2 ). By solving the PFR mass balance for A, compute kCA03/22 (c). If a single PFR (space time 3 ) is used in place of the CSTR-PFR combination, compute kCA03/23 for 80% conversion of A. Compare 3 with (1+2) and comment on the difference
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


