Question: 8a. Cerussite (P6CO3) is a secondary mineral often associated with Pb ores (i.e., galena). It is also sometimes found in old lead pipes. When
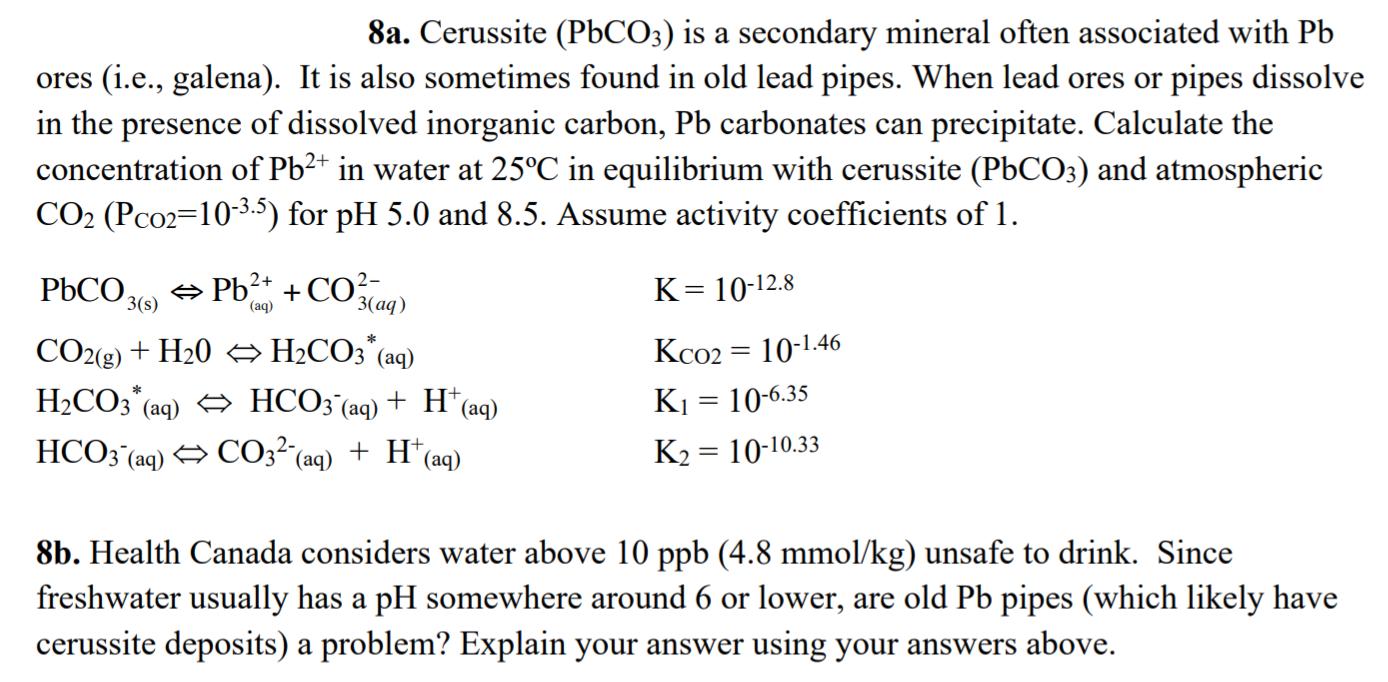
8a. Cerussite (P6CO3) is a secondary mineral often associated with Pb ores (i.e., galena). It is also sometimes found in old lead pipes. When lead ores or pipes dissolve in the presence of dissolved inorganic carbon, Pb carbonates can precipitate. Calculate the concentration of Pb2* in water at 25C in equilibrium with cerussite (PbCO3) and atmospheric CO2 (Pco2=10-3.5) for pH 5.0 and 8.5. Assume activity coefficients of 1. PbCO, O Pb2+ + CO?- K= 10-12.8 3(s) (aq) 3(aq) * Kco2 = 10-1.46 CO2(g) + H20 H2CO3"(aq) H2CO3 (aq) HCO3'(aq) + H* (aq) HCO3 (aq) CO3 (aq) K1 = 10-6.35 + H*(aq) K2 = 10-10.33 %3D 8b. Health Canada considers water above 10 ppb (4.8 mmol/kg) unsafe to drink. Since freshwater usually has a pH somewhere around 6 or lower, are old Pb pipes (which likely have cerussite deposits) a problem? Explain your answer using your answers above.
Step by Step Solution
3.39 Rating (146 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


