Question: Arrange these ions according to ionic radius. CI S- K+ Largest radius Smallest radius Answer Bank p- Ca+
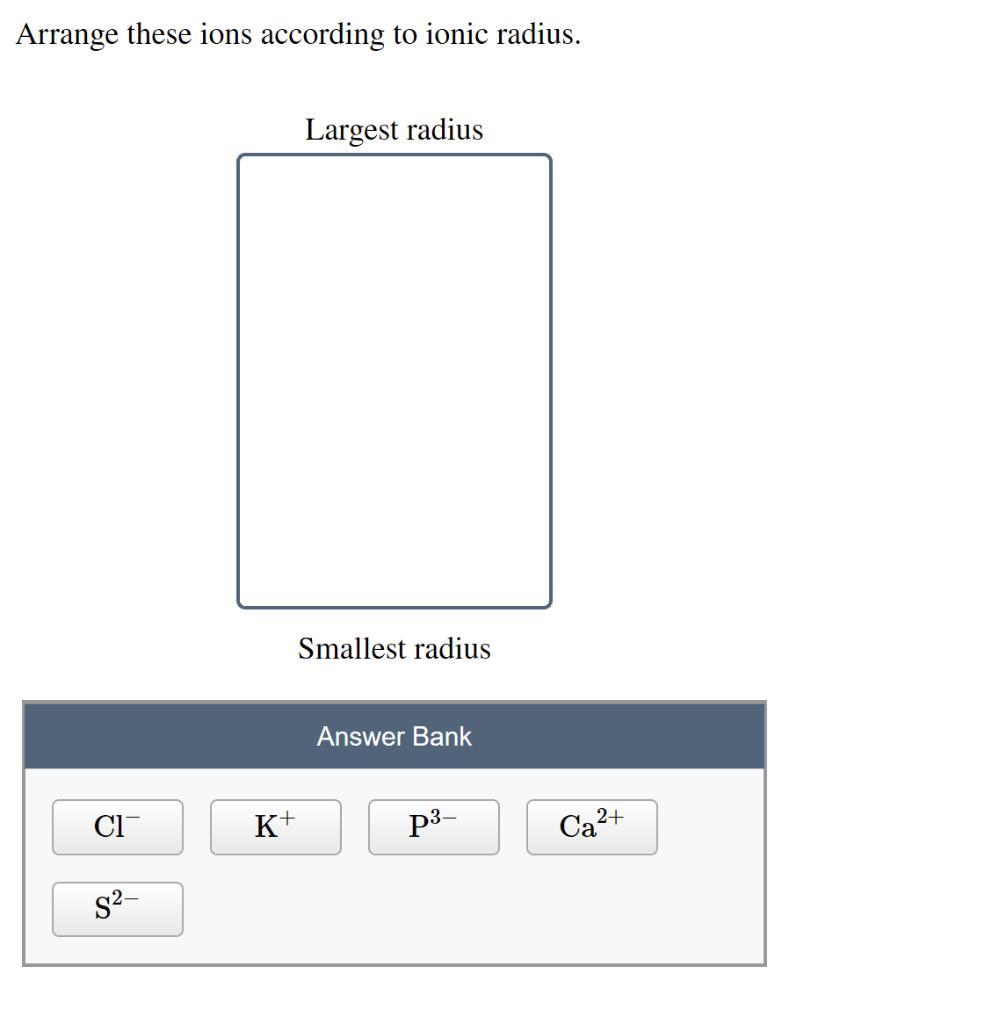
Arrange these ions according to ionic radius. CI S- K+ Largest radius Smallest radius Answer Bank p- Ca+
Step by Step Solution
★★★★★
3.33 Rating (150 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Ionic radius of a 181 pm kt ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


