Question: Arrhenius equation Rate =Ce -Q/RT C=constant (dependent on temp) Q=activation energy R=universal gasconstant T=absolute temperature In (rate) 3000 2000 1000 Intercept = In C
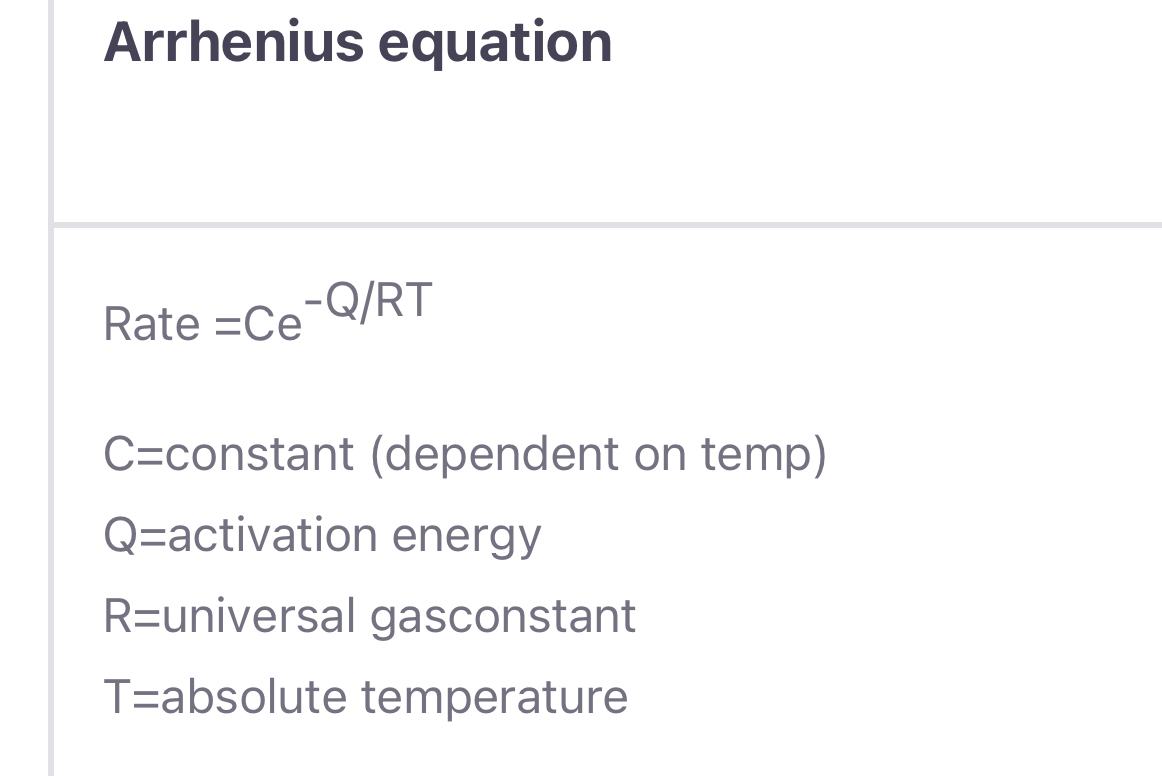
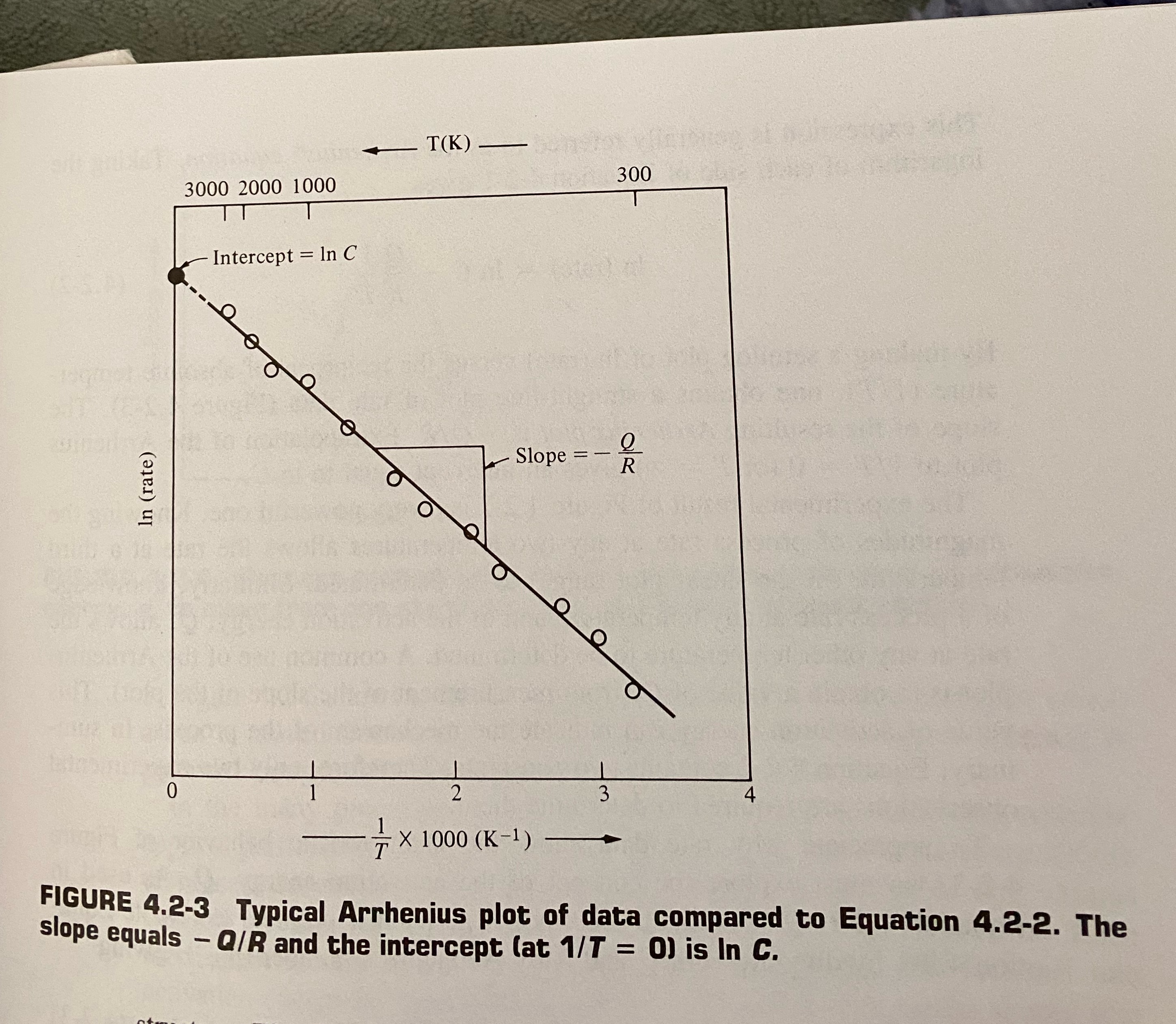
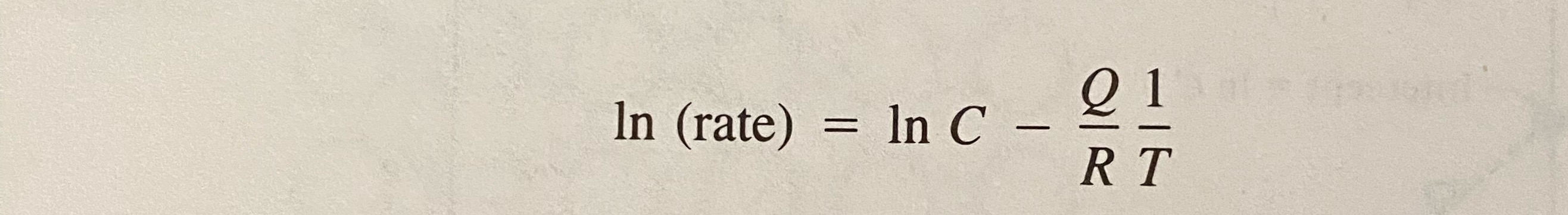
Arrhenius equation Rate =Ce -Q/RT C=constant (dependent on temp) Q=activation energy R=universal gasconstant T=absolute temperature In (rate) 3000 2000 1000 Intercept = In C T(K) tox vicino Slope = - 300 0 1 2 3 4 X 1000 (K-1) - FIGURE 4.2-3 Typical Arrhenius plot of data compared to Equation 4.2-2. The slope equals - QIR and the intercept (at 1/7 = 0) is In C. = In C - 21 RT In (rate) =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


