Question: . As a 1st year PhD student learning how to synthesize polystyrene, you try running a free radical polymerization in bulk. You mix styrene (8.07
 .
.
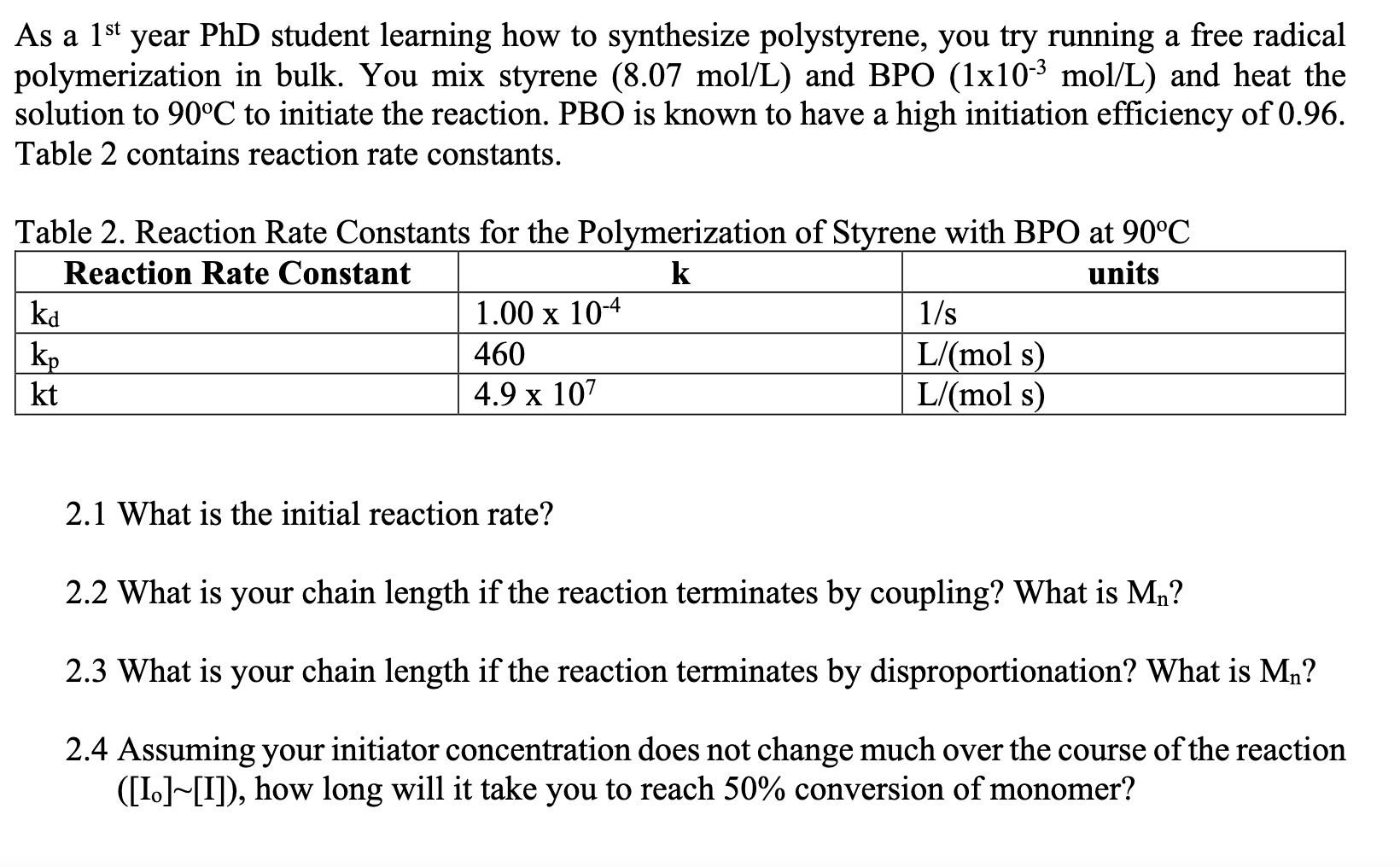
As a 1st year PhD student learning how to synthesize polystyrene, you try running a free radical polymerization in bulk. You mix styrene (8.07 mol/L) and BPO (1x10-3 mol/L) and heat the solution to 90C to initiate the reaction. PBO is known to have a high initiation efficiency of 0.96. Table 2 contains reaction rate constants. Table 2. Reaction Rate Constants for the Polymerization of Styrene with BPO at 90C Reaction Rate Constant k units ka 1.00 x 10-4 1/s kp 460 L/mol s) kt 4.9 x 107 L/(mol s) 2.1 What is the initial reaction rate? 2.2 What is your chain length if the reaction terminates by coupling? What is Mn? 2.3 What is your chain length if the reaction terminates by disproportionation? What is M,? 2.4 Assuming your initiator concentration does not change much over the course of the reaction ([1.][I]), how long will it take you to reach 50% conversion of monomer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


