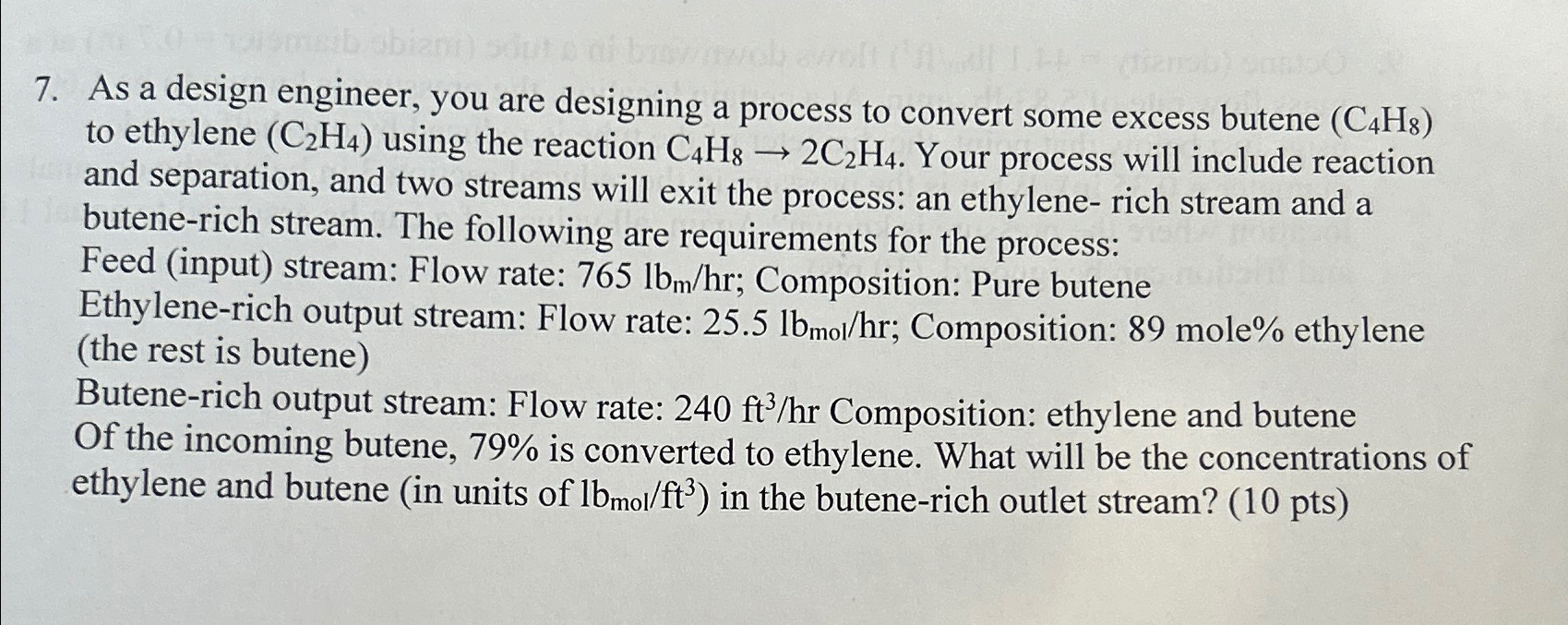
Question: As a design engineer, you are designing a process to convert some excess butene ( C 4 H 8 ) to ethylene ( C 2
As a design engineer, you are designing a process to convert some excess butene to ethylene using the reaction Your process will include reaction and separation, and two streams will exit the process: an ethylene rich stream and a butenerich stream. The following are requirements for the process:
Feed input stream: Flow rate: ; Composition: Pure butene Ethylenerich output stream: Flow rate: ; Composition: mole ethylene the rest is butene
Butenerich output stream: Flow rate: Composition: ethylene and butene Of the incoming butene, is converted to ethylene. What will be the concentrations of ethylene and butene in units of : in the butenerich outlet stream? pts
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


