Question: As a function of concentration, the exchange current density io=nFkoCRed1COx. Consider two H2H2O gas mixtures fed to a fuel cell anode at 298K : i)
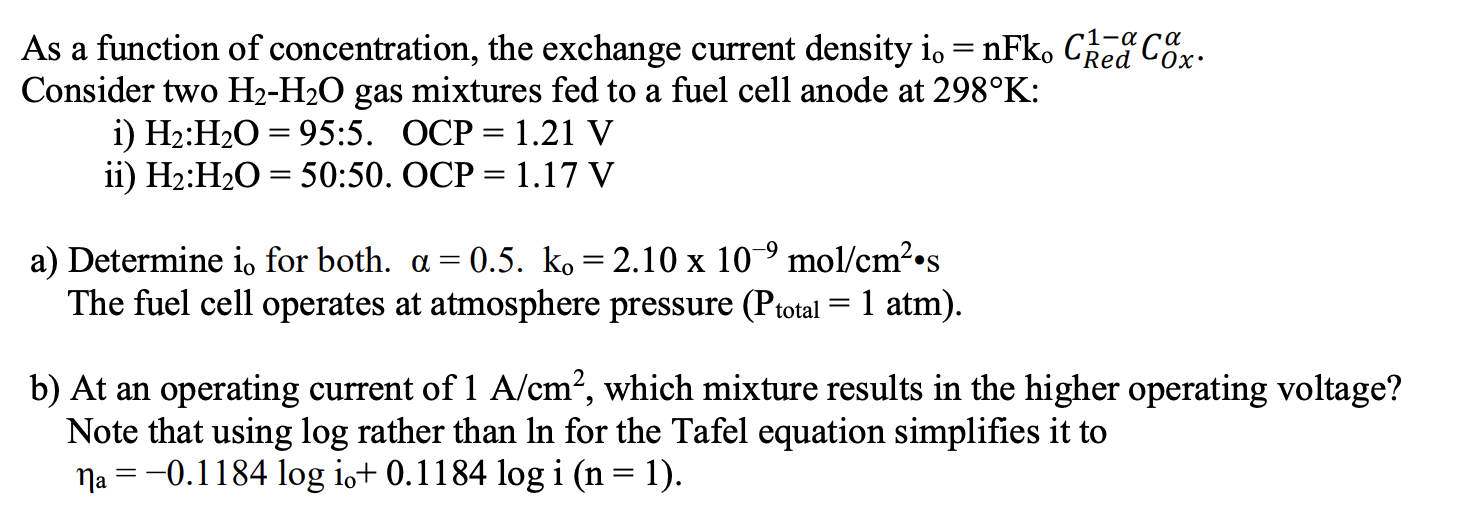
As a function of concentration, the exchange current density io=nFkoCRed1COx. Consider two H2H2O gas mixtures fed to a fuel cell anode at 298K : i) H2:H2O=95:5. OCP =1.21V ii) H2:H2O=50:50.OCP=1.17V a) Determine io for both. =0.5.ko=2.10109mol/cm2s The fuel cell operates at atmosphere pressure (Ptotal=1atm). b) At an operating current of 1A/cm2, which mixture results in the higher operating voltage? Note that using log rather than ln for the Tafel equation simplifies it to a=0.1184logio+0.1184logi(n=1)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


