Question: As shown in Figure 1, chemical C is diffusing from a bulk liquid into a sediment. The diffusivity of C in both the sediment and
As shown in Figure 1, chemical C is diffusing from a bulk liquid into a sediment. The diffusivity of C in both the sediment and water can be assumed here to be 2.42x10-6cm2/s. The chemical concentration at the sediment surface is Cs = 2 mg/L.
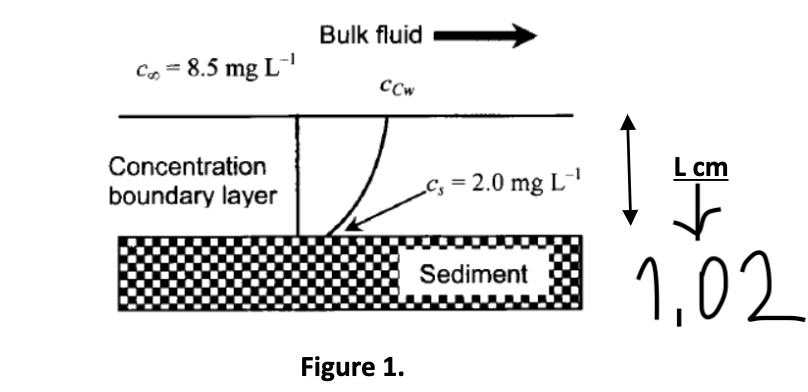
a)What is the observed liquid mass transport coefficient for this system based on a bulk concentration of chemical C =8.5mg/L?
b)What is the chemical flux into the sediment?
c)If L was multiplied by 100, how would the flux change? Explain.
Figure 1. Figure 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


