Question: As shown in the figure below, you have a system including a Uranium vessel with mass M vessel = 0.473 kg. The vessel contains water
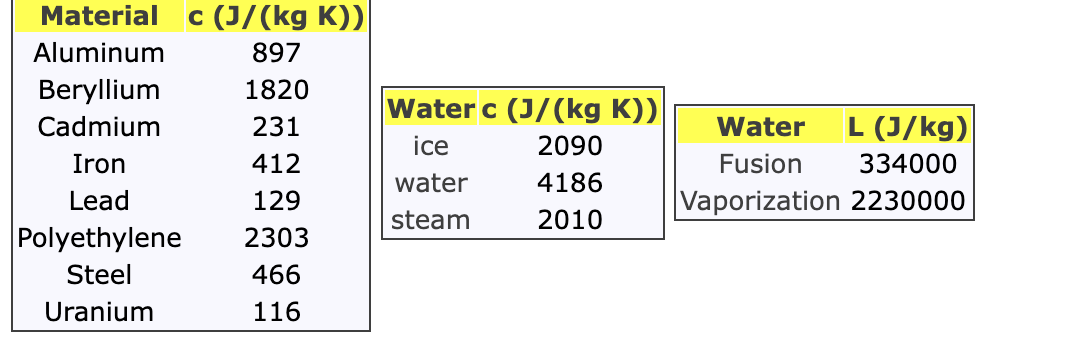
As shown in the figure below, you have a system including a Uranium vessel with mass Mvessel = 0.473 kg. The vessel contains water with mass Mwater = 0.226 kg. Both the vessel and water are initially at a temperature of 30.3 degrees Celsius. You add steam into the system. The steam has an initial temperature of 122.5 degrees Celsius and a mass of Msteam = 0.0129 kg. The system will reach equilibrium with all the steam having condensed at some final temperature below 100 degrees Celsius.
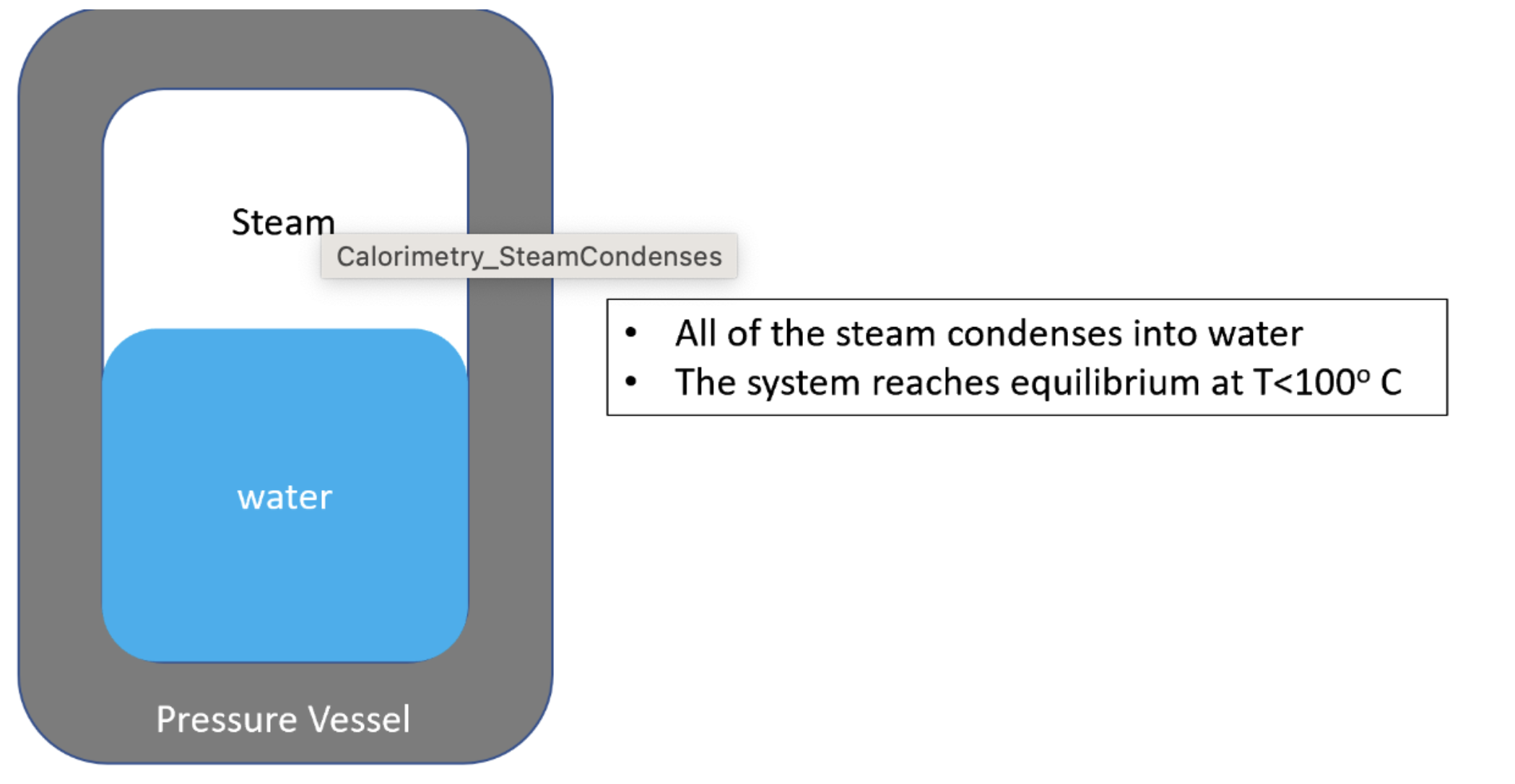
Steam Calorimetry_SteamCondenses All of the steam condenses into water The system reaches equilibrium at T
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


