Question: AS SOON AS POSSIBLE, PLEASE WRONG ANSWER=DISLIKE 1. The process of steam methane reforming is summarized by two gas-phase reactions presented below: CH4 + H2O
AS SOON AS POSSIBLE, PLEASE WRONG ANSWER=DISLIKE
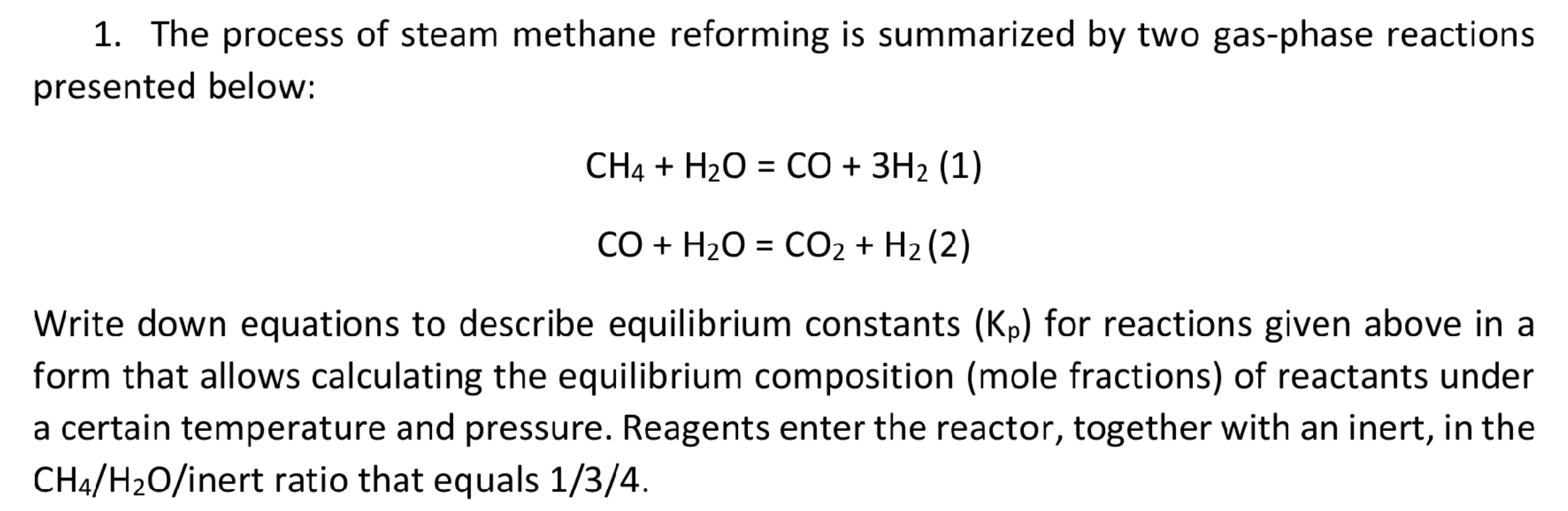
1. The process of steam methane reforming is summarized by two gas-phase reactions presented below: CH4 + H2O = CO + 3H2 (1) = CO + H2O = CO2 + H2 (2) = Write down equations to describe equilibrium constants (Kp) for reactions given above in a form that allows calculating the equilibrium composition (mole fractions) of reactants under a certain temperature and pressure. Reagents enter the reactor, together with an inert, in the CH4/H20/inert ratio that equals 1/3/4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


