Question: ASAP PLEASE!!! (i) This is a Numeric Entry question / It is worth 1 point / You have unlimited attempts / There is no attempt
ASAP PLEASE!!!
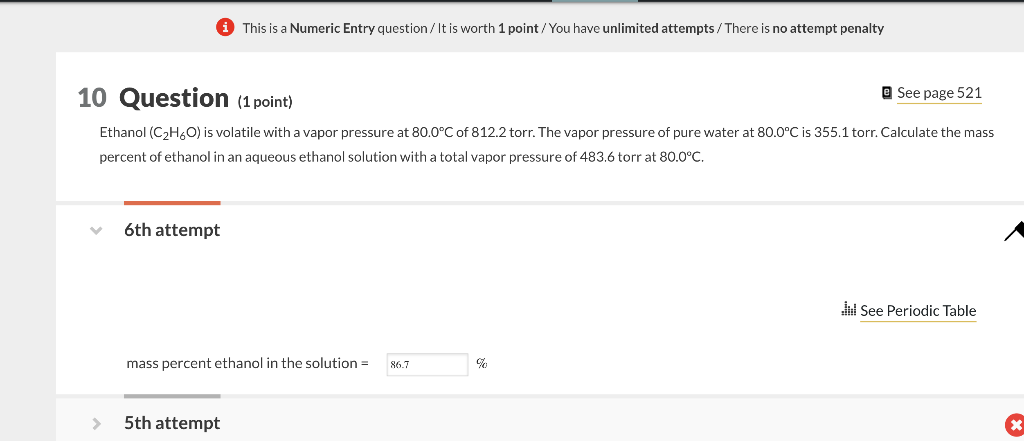
(i) This is a Numeric Entry question / It is worth 1 point / You have unlimited attempts / There is no attempt penalty 25 Question (1 point) Q See page 526 The vapor pressure of pure water is 17.50 torr at 20C, the freezing point of water is 0.00C, and the freezing point depression constant for water, Kf, is 1.86C/m. If an aqueous solution of glycerol (C3H8O3) has a vapor pressure of 13.53 torr, what would be the freezing point of the solution? 6th attempt (i) This is a Numeric Entry question / It is worth 1 point / You have unlimited attempts/ There is no attempt penalty 10 Question (1 point) See page 521 Ethanol (C2H6O) is volatile with a vapor pressure at 80.0C of 812.2 torr. The vapor pressure of pure water at 80.0C is 355.1 torr. Calculate the mass percent of ethanol in an aqueous ethanol solution with a total vapor pressure of 483.6 torr at 80.0C. 6th attempt See Periodic Table mass percent ethanol in the solution =%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


