Question: ASSAP PLEASE!!! The data provided below (Table 1) were collected for the following reaction at 904C 2NO(g) + 2H2(g) - N2(g) + 2H2O(g) Table 1:
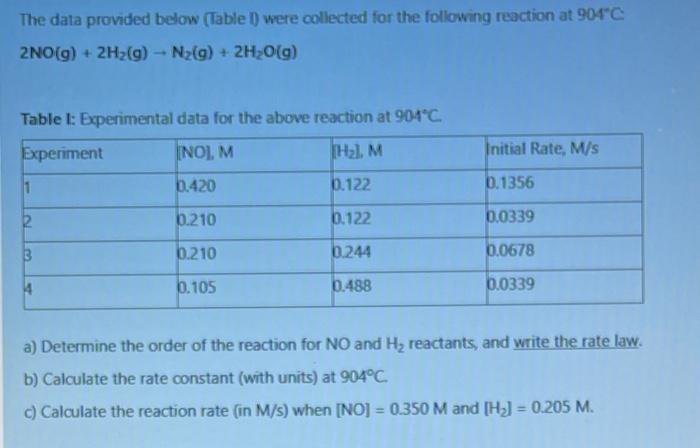
The data provided below (Table 1) were collected for the following reaction at 904C 2NO(g) + 2H2(g) - N2(g) + 2H2O(g) Table 1: Experimental data for the above reaction at 904C Experiment [NO].M THz. M Initial Rate, M/S 11 6.420 6.122 0.1356 6.210 6.122 6.0339 B 0.210 6244 0.0678 4 5.105 6.488 0.0339 a) Determine the order of the reaction for NO and Hy reactants, and write the rate law. b) Calculate the rate constant (with units) at 904C c) Calculate the reaction rate (in M/s) when (NO) = 0.350 M and [H] = 0.205 M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


