Question: Assignment Booklet B1 60. When a 0.10 mol/L solution of benzoic acid and a 0.10 mol/L solution of hydrochloric acid are tested with conductivity meters
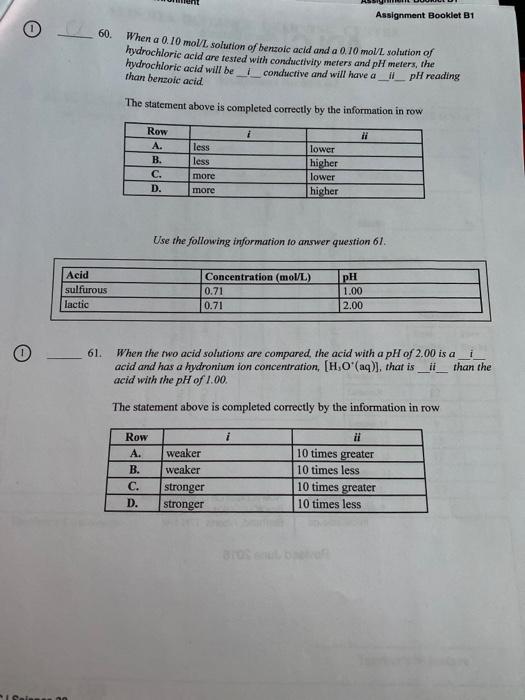
Assignment Booklet B1 60. When a 0.10 mol/L solution of benzoic acid and a 0.10 mol/L solution of hydrochloric acid are tested with conductivity meters and pH meters, the hydrochloric acid will be conductive and will have a il_pH reading than benzoic acid. The statement above is completed correctly by the information in row ii Row A. B. C. D. less less lower higher lower higher more more Use the following information to answer question 61. Acid sulfurous lactic Concentration (moVL) 0.71 0.71 pH 1.00 2.00 61. When the two acid solutions are compared the acid with a pH of 2.00 is a di acid and has a hydronium ion concentration, (H.O'(aq)], that is il than the acid with the pH of 1.00 The statement above is completed correctly by the information in row Row A. B. C. D. weaker weaker stronger stronger ii 10 times greater 10 times less 10 times greater 10 times less
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


