Question: assume pressure is constant, pls need answer w numbers Consider the combustion of a fuel and oxidizer in an adiabatic plug-flow reactor of constant cross-sectional
 assume pressure is constant, pls need answer w numbers
assume pressure is constant, pls need answer w numbers
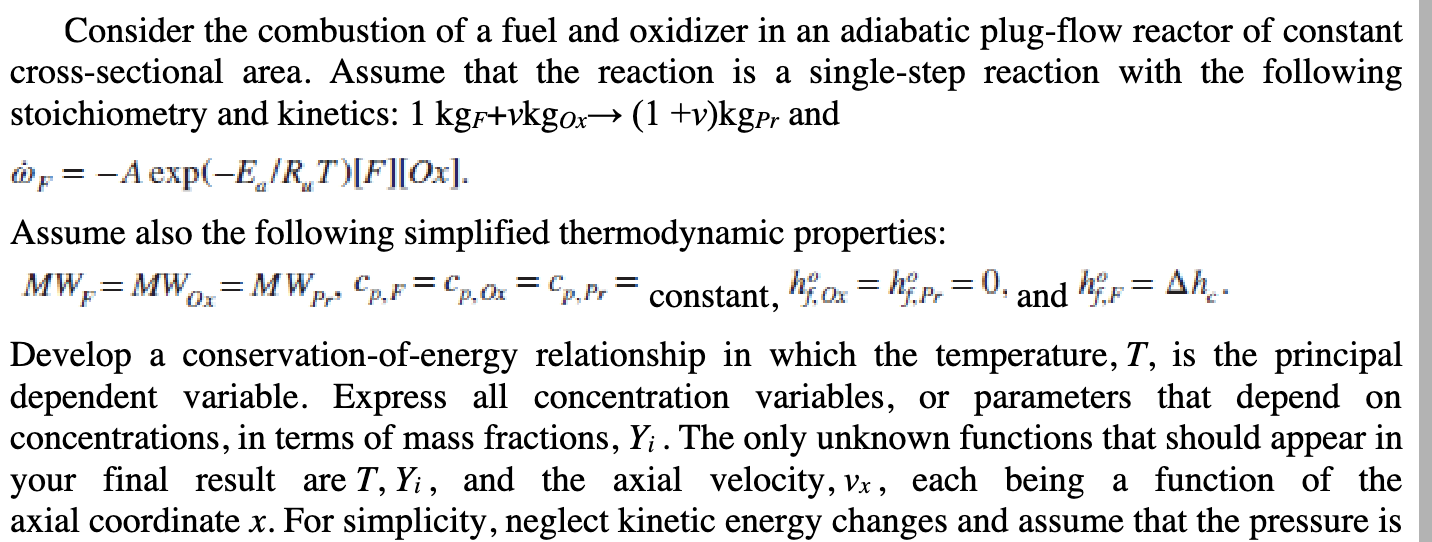
Consider the combustion of a fuel and oxidizer in an adiabatic plug-flow reactor of constant cross-sectional area. Assume that the reaction is a single-step reaction with the following stoichiometry and kinetics: 1kgF+vkgOx(1+v)kgPr and F=Aexp(Ea/RwT)[F][Ox] Assume also the following simplified thermodynamic properties: MWF=MWOx=MWPr,cp,F=cp,Ox=cp,Pr=constant,hf,Oxo=hf,Pro=0,andhf,Fv=hc. Develop a conservation-of-energy relationship in which the temperature, T, is the principal dependent variable. Express all concentration variables, or parameters that depend on concentrations, in terms of mass fractions, Yi. The only unknown functions that should appear in your final result are T,Yi, and the axial velocity, vx, each being a function of the axial coordinate x. For simplicity, neglect kinetic energy changes and assume that the pressure is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


