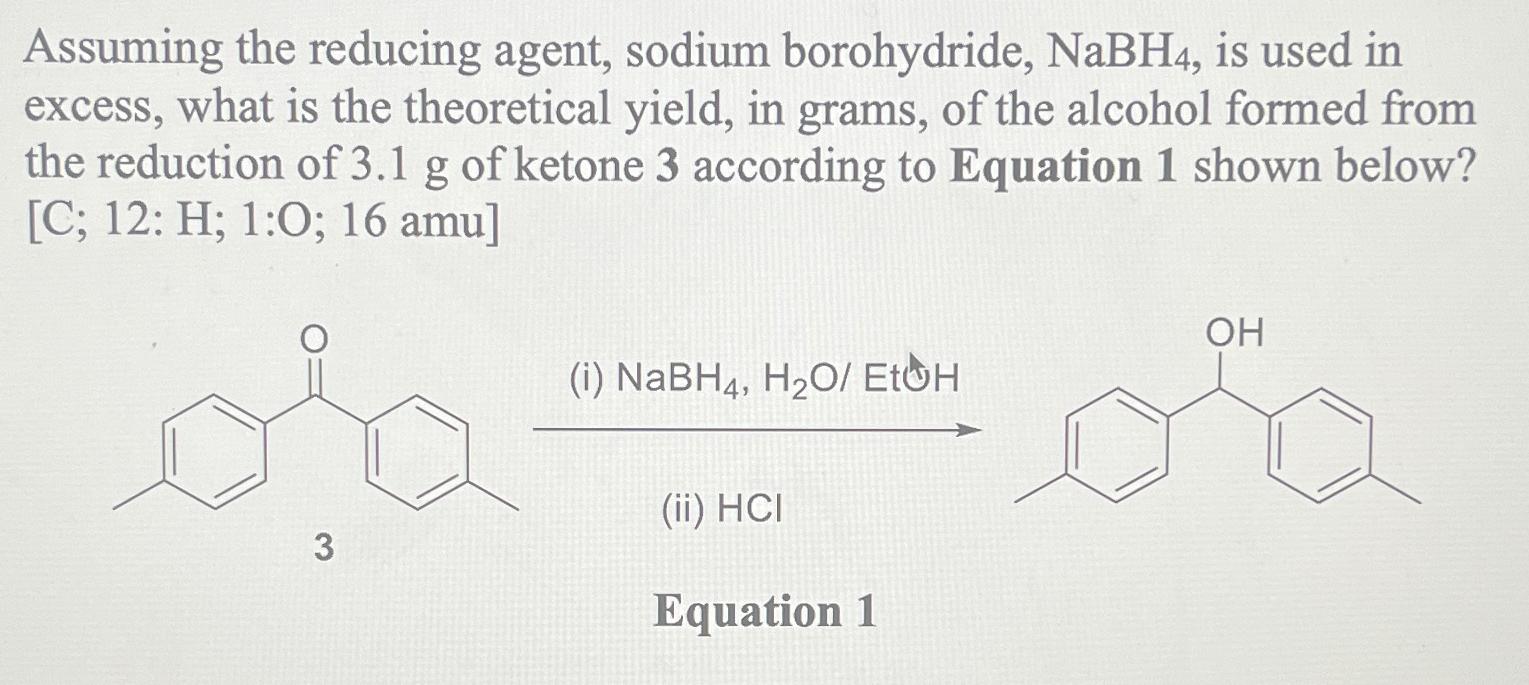
Question: Assuming the reducing agent, sodium borohydride, N a B H 4 , is used in excess, what is the theoretical yield, in grams, of the
Assuming the reducing agent, sodium borohydride, is used in excess, what is the theoretical yield, in grams, of the alcohol formed from the reduction of of ketone according to Equation shown below? ;:;:;
i
ii
Equation
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


