Question: At -5.49 C the pressure equilibrium constant K = 7.8 x 10 for a certain reaction. Here are some facts about the reaction: Some of
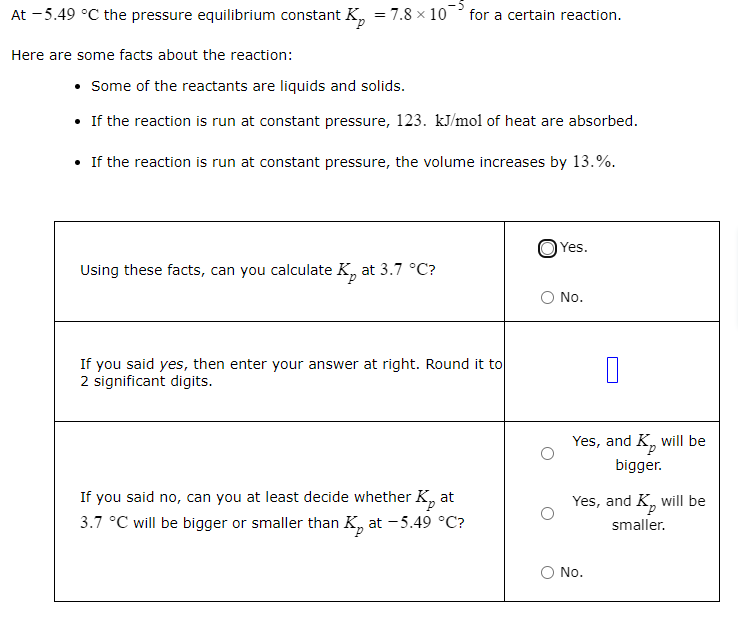
At -5.49 C the pressure equilibrium constant K = 7.8 x 10 for a certain reaction. Here are some facts about the reaction: Some of the reactants are liquids and solids. If the reaction is run at constant pressure, 123. kJ/mol of heat are absorbed. If the reaction is run at constant pressure, the volume increases by 13.%. OYes. Using these facts, can you calculate K, at 3.7 C? O No. If you said yes, then enter your answer at right. Round it to 2 significant digits. Yes, and K, will be bigger. Yes, and K, will be smaller. If you said no, can you at least decide whether K, at 3.7 C will be bigger or smaller than K, at -5.49 C? O No
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


