Question: Problem 1: Consider the following reaction: A+BC+D a) What is the correct expression for the equilibrium constant (k) ? b) The k value for this
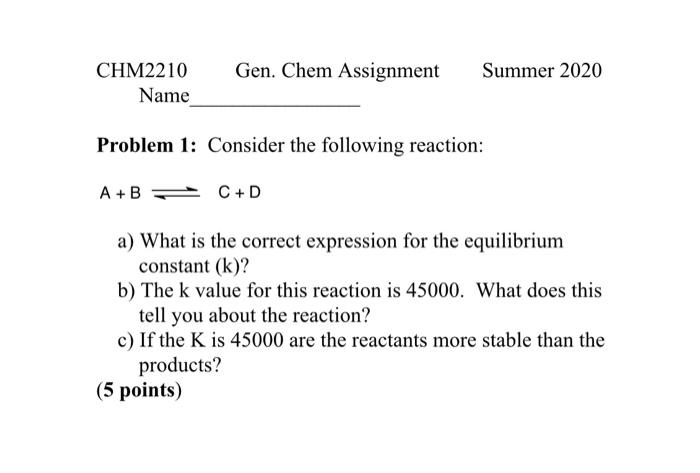


Problem 1: Consider the following reaction: A+BC+D a) What is the correct expression for the equilibrium constant (k) ? b) The k value for this reaction is 45000 . What does this tell you about the reaction? c) If the K is 45000 are the reactants more stable than the products? (5 points) Problem 3: Consider the following reaction: A+BC+D According to Le Chatelier's Principle what would happen if you added more D to the reaction? Explain your answer from the point of view of what is happening with the molecules. Do not simply state that an equilibrium under pressure responds in a certain way. That literally means nothing. (5 points) Problem 4: Consider the following reaction: A+BC+D The rate of this reaction is decreasing as products form. Is this statement true or false? Explain your answer. Your answer may use a maximum of 15 words. Using more than 15 words will result in a 0 score. (5 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


