Question: At a certain temperature, A (M= 34 g/mol) decomposes into R and L in the gas phase. The reaction is second order with respect to
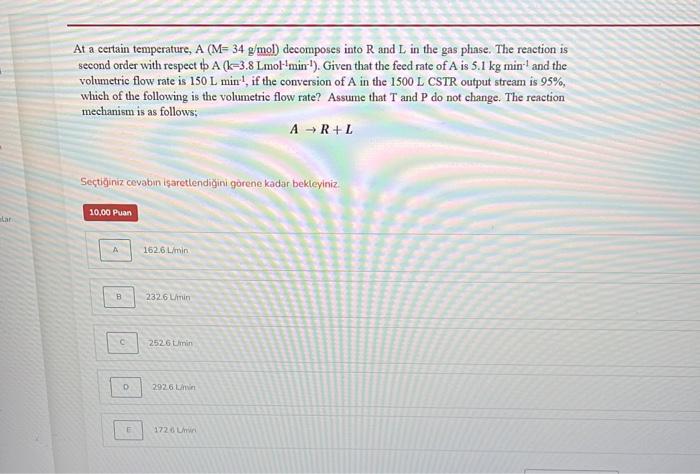
At a certain temperature, A(M=34g/mol) decomposes into R and L in the gas phase. The reaction is second order with respect bA(k=3.8Lmol1min1). Given that the feed rate of A is 5.1kgmin1 and the volumetric flow rate is 150Lmin1, if the conversion of A in the 1500L CSTR output stream is 95%, which of the following is the volumetric flow rate? Assume that T and P do not change. The reaction mechanism is as follows: AR+L Sectiginiz cevabun iaretlendigini gorene kadar bekleyiniz 162.6Limin 232.6 Uiin 2526 tinin 2926 t.min 1726 Unini
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


