Question: At a certain temperature, Kc = 2.0 for the following reaction. HOCN(8)+ H2O() H30+(g) + OCN (9) A 0.75 L reaction vessel contained 0.45 mol
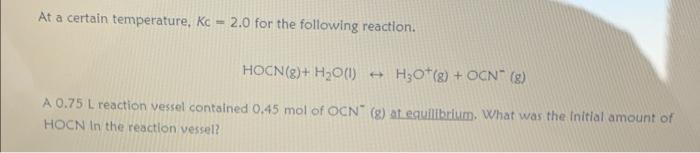
At a certain temperature, Kc = 2.0 for the following reaction. HOCN(8)+ H2O() H30+(g) + OCN (9) A 0.75 L reaction vessel contained 0.45 mol of OCN (g) at equilibrium. What was the initial amount of HOCN In the reaction vessel
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


