Question: At a certain temperature, the equilibrium partial pressures for the substances in the reaction 2 N205 (g) 02 (g) +4 NO2 (g) were: PN205=3.21
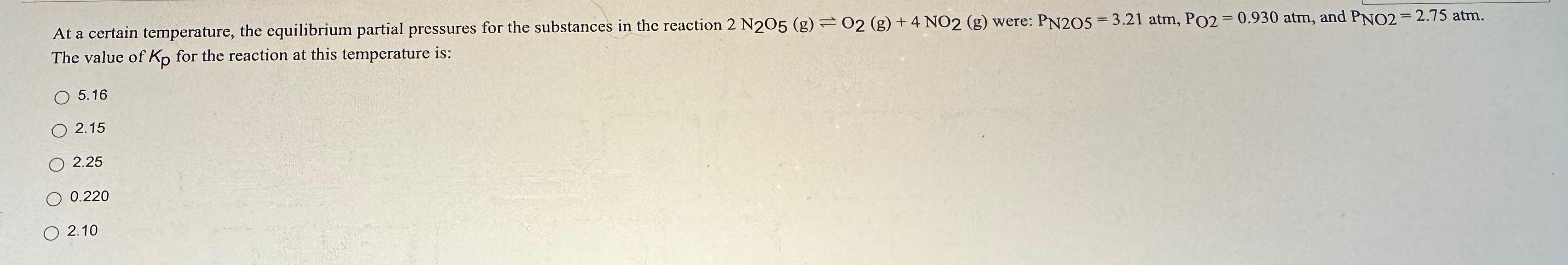
At a certain temperature, the equilibrium partial pressures for the substances in the reaction 2 N205 (g) 02 (g) +4 NO2 (g) were: PN205=3.21 atm, PO2 = 0.930 atm, and PNO2 = 2.75 atm. The value of Kp for the reaction at this temperature is: 5.16 2.15 2.25 0.220 2.10
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


