Question: At a certain temperature the following data were collected for the reaction 2ICl+H2I2+2HCl. Intial Concentrations (mol. L1 ) Determine the rate law and the rate
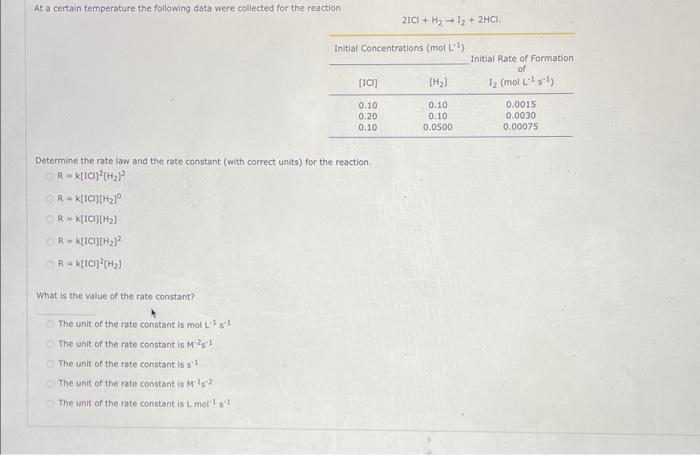
At a certain temperature the following data were collected for the reaction 2ICl+H2I2+2HCl. Intial Concentrations (mol. L1 ) Determine the rate law and the rate constant (with correct units) for the reaction. R=k[tCl]2[H2]2R=k[tCl][H2]0R=k[ICl][H2]R=k[ICl][H2]2R=k[ICl]2[H2] What is the value of the rate constant? The unit of the rate constant is mol L1s1 The unit of the rate constant is M2g1. The unit of the rate constant is s1 The unit of the rate constant is M1s2 The unit of the rate constant is Lmol1s1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


