Question: At a certain temperature, the pH of a neutral solution is 7.24. What is the value of Kw at that temperature? Express your answer numerically
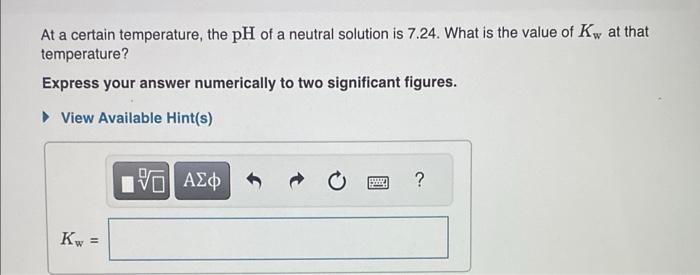
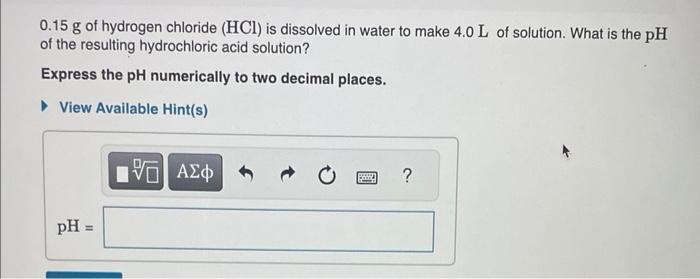
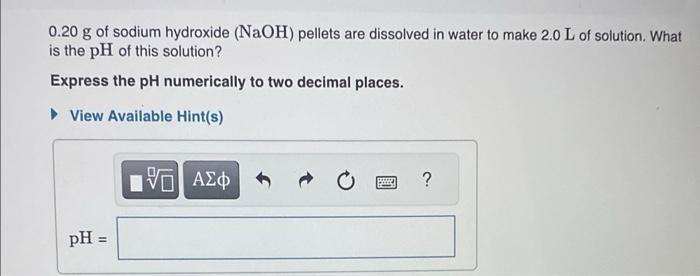
At a certain temperature, the pH of a neutral solution is 7.24. What is the value of Kw at that temperature? Express your answer numerically to two significant figures. 0.15g of hydrogen chloride (HCl) is dissolved in water to make 4.0L of solution. What is the pH of the resulting hydrochloric acid solution? Express the pH numerically to two decimal places. 0.20g of sodium hydroxide (NaOH) pellets are dissolved in water to make 2.0L of solution. What is the pH of this solution? Express the pH numerically to two decimal places
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


