Question: At a certain temperature, the pH of a neutral solution is 7.26. What is the value of Kw at that temperature? Express your answer numerically
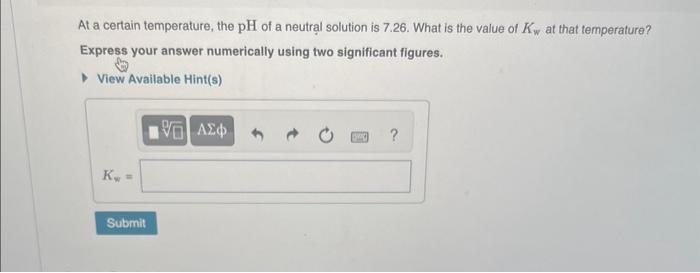
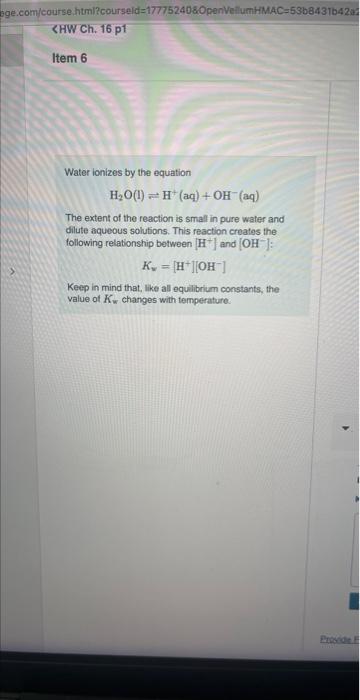
At a certain temperature, the pH of a neutral solution is 7.26. What is the value of Kw at that temperature? Express your answer numerically using two significant figures. View Available Hint(s) Water ionizes by the equation H2O(l)H+(aq)+OH(aq) The extent of the reaction is small in pure water and dilute aqueous solutions. This reaction creates the following relationship between [H+]and OH]: Kw=[H+][OH] Keep in mind that, like all equilibrium constants, the value of Kw changes with temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


