Question: Questions 1 & 2 refer to the solubility of iron(II) phosphate, Fe3(PO4)2: Fe3(PO4)2(s) 3Fe+ (aq) + 2PO4 (aq) 1. The Ksp for Fe3(PO4)2(s) is
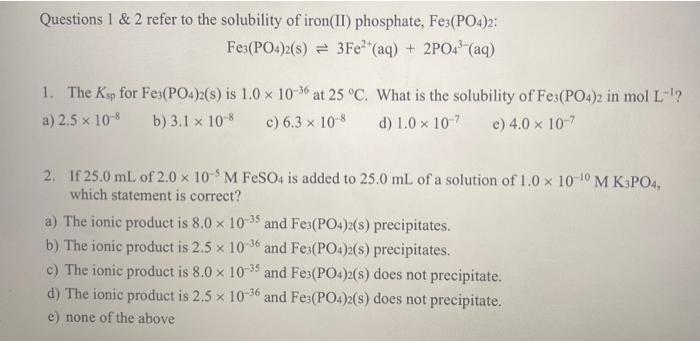
Questions 1 & 2 refer to the solubility of iron(II) phosphate, Fe3(PO4)2: Fe3(PO4)2(s) 3Fe+ (aq) + 2PO4 (aq) 1. The Ksp for Fe3(PO4)2(s) is 1.0 x 10-36 at 25 C. What is the solubility of Fe3(PO4)2 in mol L-? a) 2.5 10-8 b) 3.1 x 10-8 c) 6.3 10-8 d) 1.0 x 10-7 e) 4.0 x 10-7 2. If 25.0 mL of 2.0 x 10-5 M FeSO4 is added to 25.0 mL of a solution of 1.0 x 10-10 M K3PO4, which statement is correct? a) The ionic product is 8.0 x 10-35 and Fe3(PO4)2(s) precipitates. b) The ionic product is 2.5 x 10-36 and Fe3(PO4)2(s) precipitates. c) The ionic product is 8.0 x 10-35 and Fe3(PO4)2(s) does not precipitate. d) The ionic product is 2.5 x 10-36 and Fe3(PO4)2(s) does not precipitate. e) none of the above
Step by Step Solution
3.32 Rating (152 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


