Question: At which pH will a solution of proline have equal concentrations of its fully protonated species and zwitterion species, [H2AA+] = [HAA] pKa1 = 1.99
At which pH will a solution of proline have equal concentrations of its fully protonated species and zwitterion species, [H2AA+] = [HAA]
pKa1 = 1.99 pKa2 = 10.96 pI = 6.48
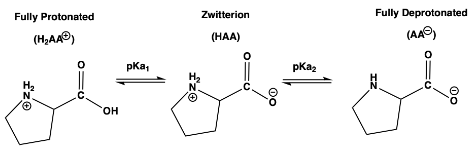
Fully Protonated Zwitterion Fully Deprotonated (H2AA) (HAA) (AA) pKa1 pKa2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


