Question: ATOMIC STRUCTURE Homework# 3 Name 1. Define the following: a. atomic number b. mass number - c. isotope - 2. What is the atomic number
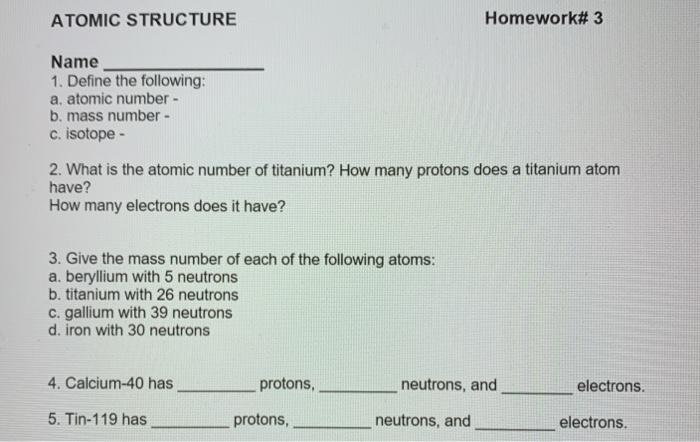
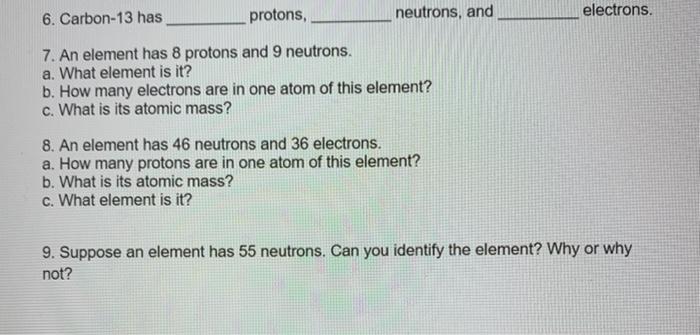
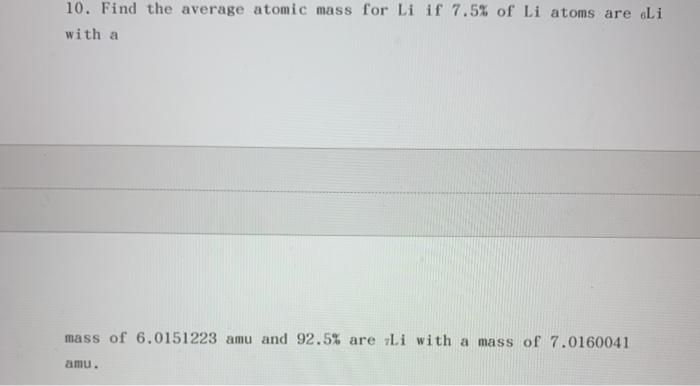

ATOMIC STRUCTURE Homework# 3 Name 1. Define the following: a. atomic number b. mass number - c. isotope - 2. What is the atomic number of titanium? How many protons does a titanium atom have? How many electrons does it have? 3. Give the mass number of each of the following atoms: a. beryllium with 5 neutrons b. titanium with 26 neutrons c. gallium with 39 neutrons d. iron with 30 neutrons 4. Calcium-40 has protons, neutrons, and electrons. 5. Tin-119 has protons, neutrons, and electrons. protons, 6. Carbon-13 has_ electrons. neutrons, and 7. An element has 8 protons and 9 neutrons. a. What element is it? b. How many electrons are in one atom of this element? c. What is its atomic mass? 8. An element has 46 neutrons and 36 electrons. a. How many protons are in one atom of this element? b. What is its atomic mass? c. What element is it? 9. Suppose an element has 55 neutrons. Can you identify the element? Why or why not? 10. Find the average atomic mass for Li if 7.5% of Li atoms are eli with a mass of 6.0151223 amu and 92.5% are Li with a mass of 7.0160041 amu. 11. Rubidium has two common isotopes, ssRb and v7Rb. If the abundance of 85Rb is 72.2% and the abundance of B7Rb is 27.8%, what is the average atomic mass of rubidium? 12. Find the average atomic mass for ci if 75.78% of Ci atoms are 35C1 with a mass of 34.96885271 amu and 24.22% are 1C1 with a mass of 36.96590260 amu
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


