Question: B. Explain why dimensional quantities like Planck's constant cannot be arguments of some functions like the exponential function eh Planck's constant has units [kg][m]*
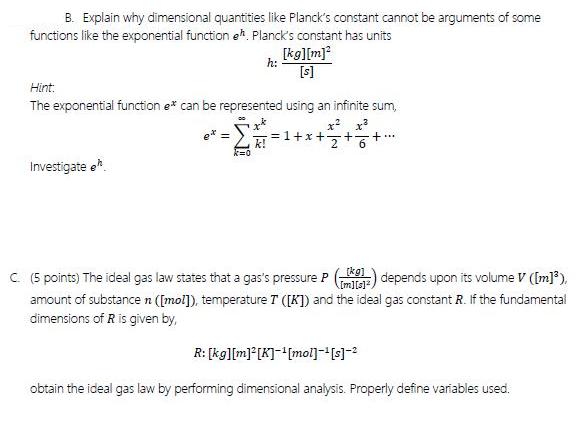
B. Explain why dimensional quantities like Planck's constant cannot be arguments of some functions like the exponential function eh Planck's constant has units [kg][m]* h: [s] Hint. The exponential function e* can be represented using an infinite sum, =1+x++6+* k! Investigate o. C (5 points) The ideal gas law states that a gas's pressure P ) depends upon its volume V (Im]), (kg] Im]is] amount of substance n ([mol]), temperature T ([K]) and the ideal gas constant R. If the fundamental dimensions of R is given by, R: [kg][m][K]-[mol] obtain the ideal gas law by performing dimensional analysis. Properly define variables used.
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
B We hmow 4 3 K0 RK 1 3 i for et t 31 21 K20 Dimenzion of ard torn of the arc... View full answer

Get step-by-step solutions from verified subject matter experts


