Question: b) Below is an irreversible first order and second order reaction in series: ky k, A R + 2S The reaction rate constant for the
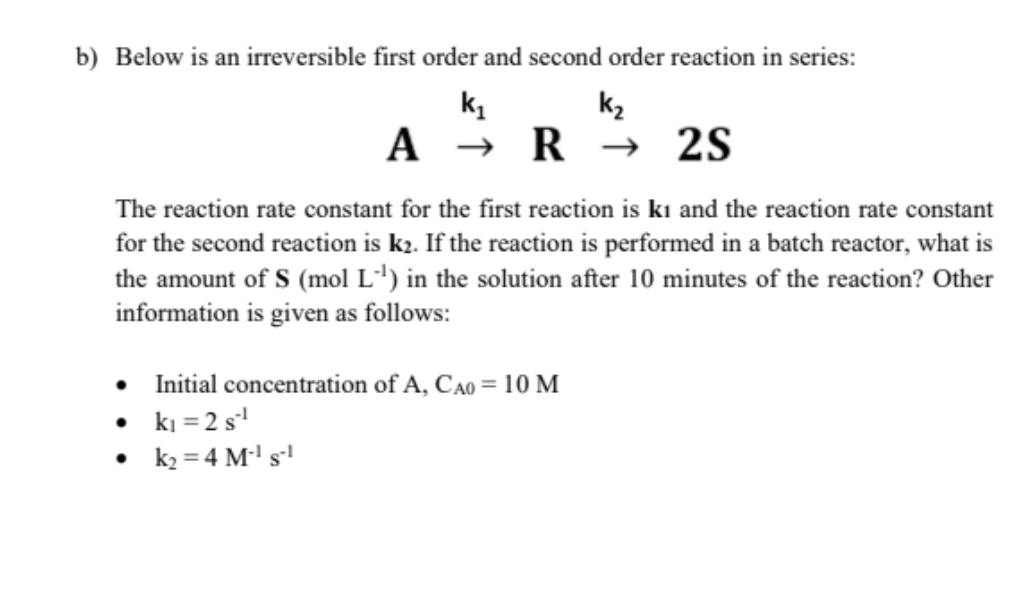
b) Below is an irreversible first order and second order reaction in series: ky k, A R + 2S The reaction rate constant for the first reaction is ki and the reaction rate constant for the second reaction is k2. If the reaction is performed in a batch reactor, what is the amount of S (mol L') in the solution after 10 minutes of the reaction? Other information is given as follows: Initial concentration of A, Cao = 10 M ki = 2 s? k2 = 4 M's b) Below is an irreversible first order and second order reaction in series: ky k, A R + 2S The reaction rate constant for the first reaction is ki and the reaction rate constant for the second reaction is k2. If the reaction is performed in a batch reactor, what is the amount of S (mol L') in the solution after 10 minutes of the reaction? Other information is given as follows: Initial concentration of A, Cao = 10 M ki = 2 s? k2 = 4 M's
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


