Question: b' Calculate the enriching operating line and plot it on in the x-y diagram c, Calculate the q number and plot the q line in
b' Calculate the enriching operating line and plot it on in the x-y diagram
c, Calculate the q number and plot the q line in the same figure as in question 2(b).
(d) () In the above figure, construct the stripping operating line based on the previous results. Estimate the number of theoretical trays needed. Plot out your estimation steps in the figure or write down the calculation steps here.
(e) () Estimate the minimum number of trays needed if total reflux is assumed
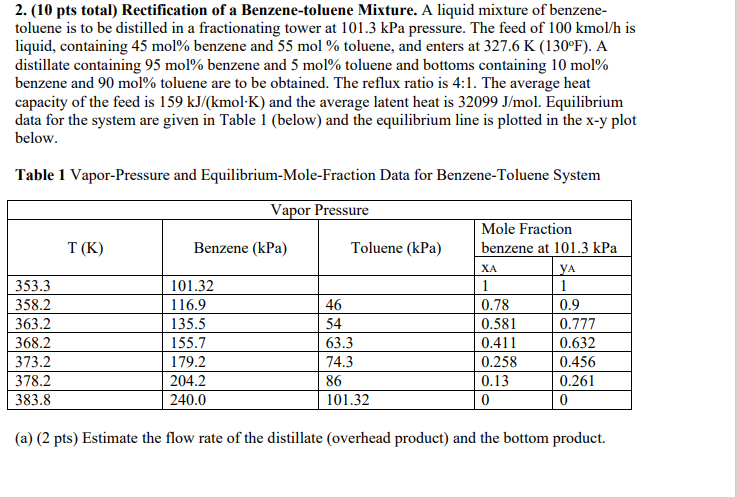
2. (10 pts total) Rectification of a Benzene-toluene Mixture. A liquid mixture of benzene- toluene is to be distilled in a fractionating tower at 101.3 kPa pressure. The feed of 100 kmol/h is liquid, containing 45 mol% benzene and 55 mol % toluene, and enters at 327.6 K (130F). A distillate containing 95 mol% benzene and 5 mol% toluene and bottoms containing 10 mol% benzene and 90 mol% toluene are to be obtained. The reflux ratio is 4:1. The average heat capacity of the feed is 159 kJ/(kmol-K) and the average latent heat is 32099 J/mol. Equilibrium data for the system are given in Table 1 (below) and the equilibrium line is plotted in the x-y plot below. XA Table 1 Vapor-Pressure and Equilibrium-Mole-Fraction Data for Benzene-Toluene System Vapor Pressure Mole Fraction T(K) Benzene (kPa) Toluene (kPa) benzene at 101.3 kPa YA 353.3 101.32 1 1 358.2 116.9 46 0.78 0.9 363.2 135.5 54 0.581 0.777 368.2 155.7 63.3 0.411 0.632 373.2 179.2 74.3 0.258 0.456 378.2 204.2 86 0.13 0.261 383.8 240.0 101.32 0 0 (a) (2 pts) Estimate the flow rate of the distillate (overhead product) and the bottom product
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


