Question: (b) Determine the bubblepoint and the equilibrium composition at 760mmHg for a liquid that consists of 9.66%mol ethanol through both Margules and van Lar equations.

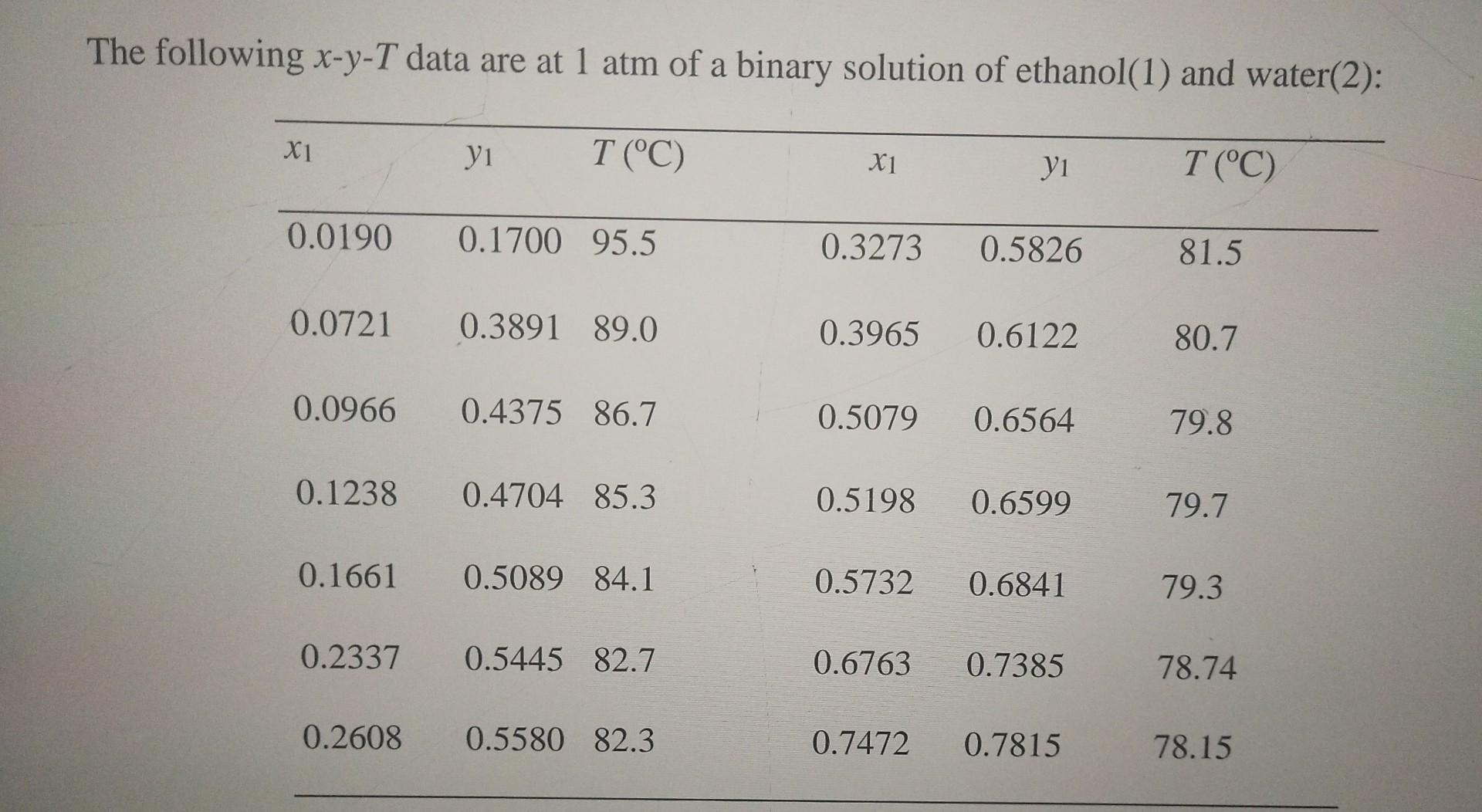
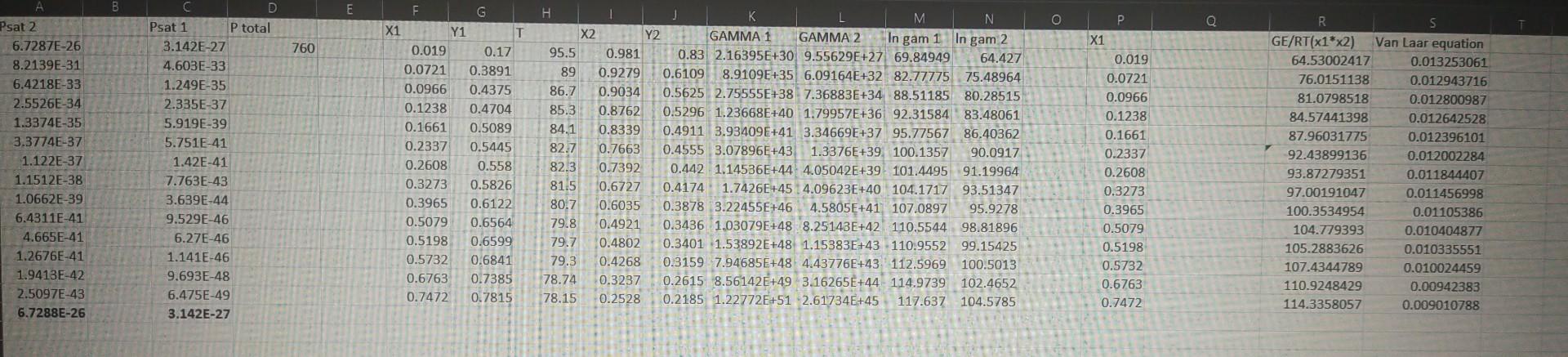
(b) Determine the bubblepoint and the equilibrium composition at 760mmHg for a liquid that consists of 9.66%mol ethanol through both Margules and van Lar equations. (c) Determine the dewpoint and the equilibrium composition at 760mmHg for a vapour that consists of 61.22%mol ethanol through both Margules and van Laar equations. (d) Determine the total pressure and the vapour composition for a mixture of 50%mol at 25C through both Margules and van Laar equations. The following xyT data are at 1atm of a binary solution of ethanol(1) and water(2): (b) Determine the bubblepoint and the equilibrium composition at 760mmHg for a liquid that consists of 9.66%mol ethanol through both Margules and van Lar equations. (c) Determine the dewpoint and the equilibrium composition at 760mmHg for a vapour that consists of 61.22%mol ethanol through both Margules and van Laar equations. (d) Determine the total pressure and the vapour composition for a mixture of 50%mol at 25C through both Margules and van Laar equations. The following xyT data are at 1atm of a binary solution of ethanol(1) and water(2)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


